rom 702 3. BALANCE the following equation: KCIO3 -------> KCI + O, a) How many grams of KCl are produced from 250 grams mole of KCIO;? b) How many grams of KCIO; are needed to produce 15 grams mole of O,?
rom 702 3. BALANCE the following equation: KCIO3 -------> KCI + O, a) How many grams of KCl are produced from 250 grams mole of KCIO;? b) How many grams of KCIO; are needed to produce 15 grams mole of O,?
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
Transcribed Image Text:O Mass-Mass Stoich-$1 doc Sc
urses/216161/files/236134137wrap31
Met-GUY, KLE L on
> Files > Mass-Mass Stoich -s-1.doc
-Mass Stoich -s-1.doc
d Mass-Mass Stoich -s-1.doc (31 KB)
700M
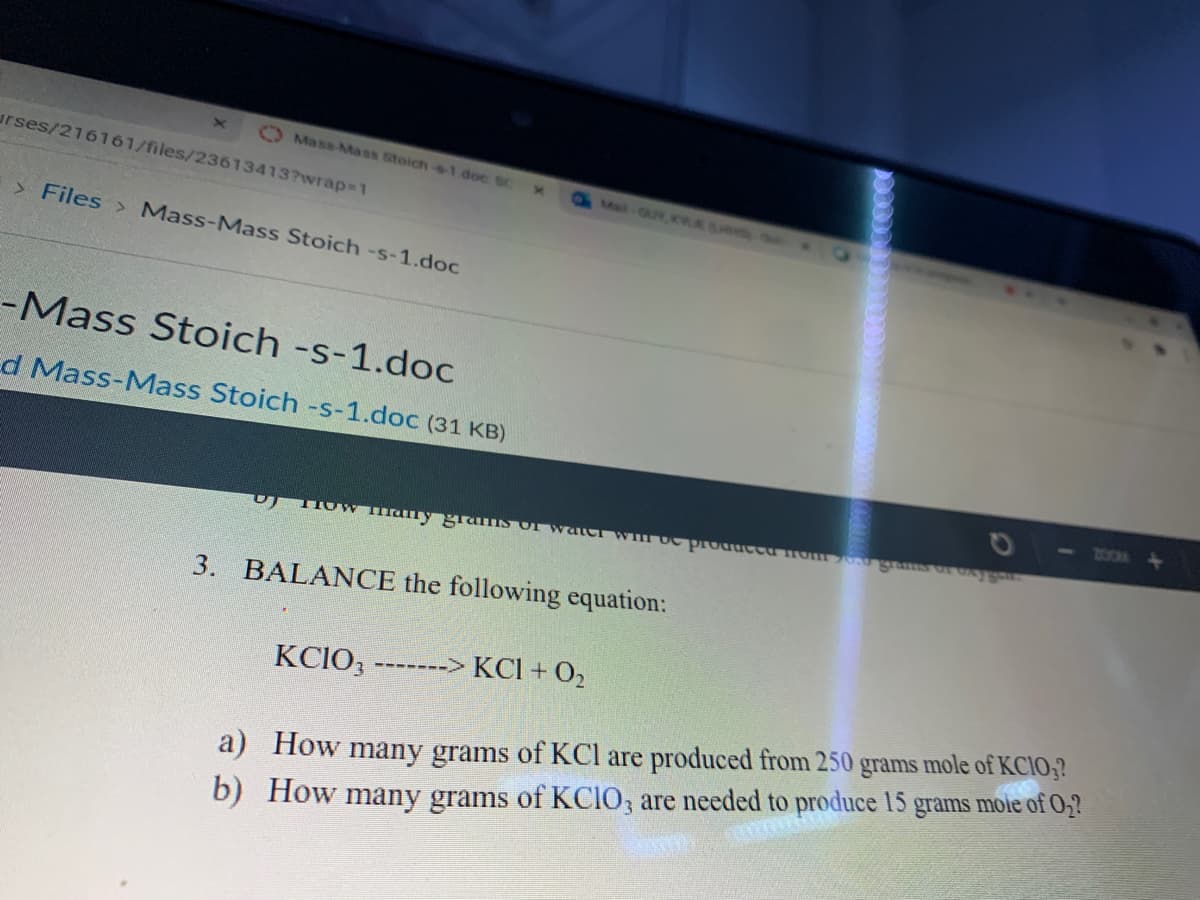
3.
BALANCE the following equation:
KCIO3
-------> KCl + O,
grams
mole of KCIO,?
a) How many grams of KCl are produced from 250
b) How many grams of KCIO, are needed to produce 15 grams mole of O;?
Expert Solution
Balancing the decomposition reaction of KClO3:
Potassium chlorate decomposes to give potassium chloride and oxygen gas:
2 KClO3
Note: The unit gram mole means molar mass of molecule/atom/ions etc in grams.
1 gram mole = mass of one mole of molecule in grams.
Calculating the mole ratio of KClO3 and KCl
Since, 2 mol of KClO3 gives 2 mol of KCl,
hence, mole ratio of KClO3 : KCl = 2 : 1
Calculating the mole ratio of KClO3 to O2 gas:
Since, 2 mol of KClO3 gives 3 mol of O2 ,
hence, mole ratio of KClO3 : O2 = 2 : 3
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning