Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 74QAP: Magnesium ribbon reacts with acid to produce hydro- gen gas and magnesium ions. Different masses of...
Related questions
Question
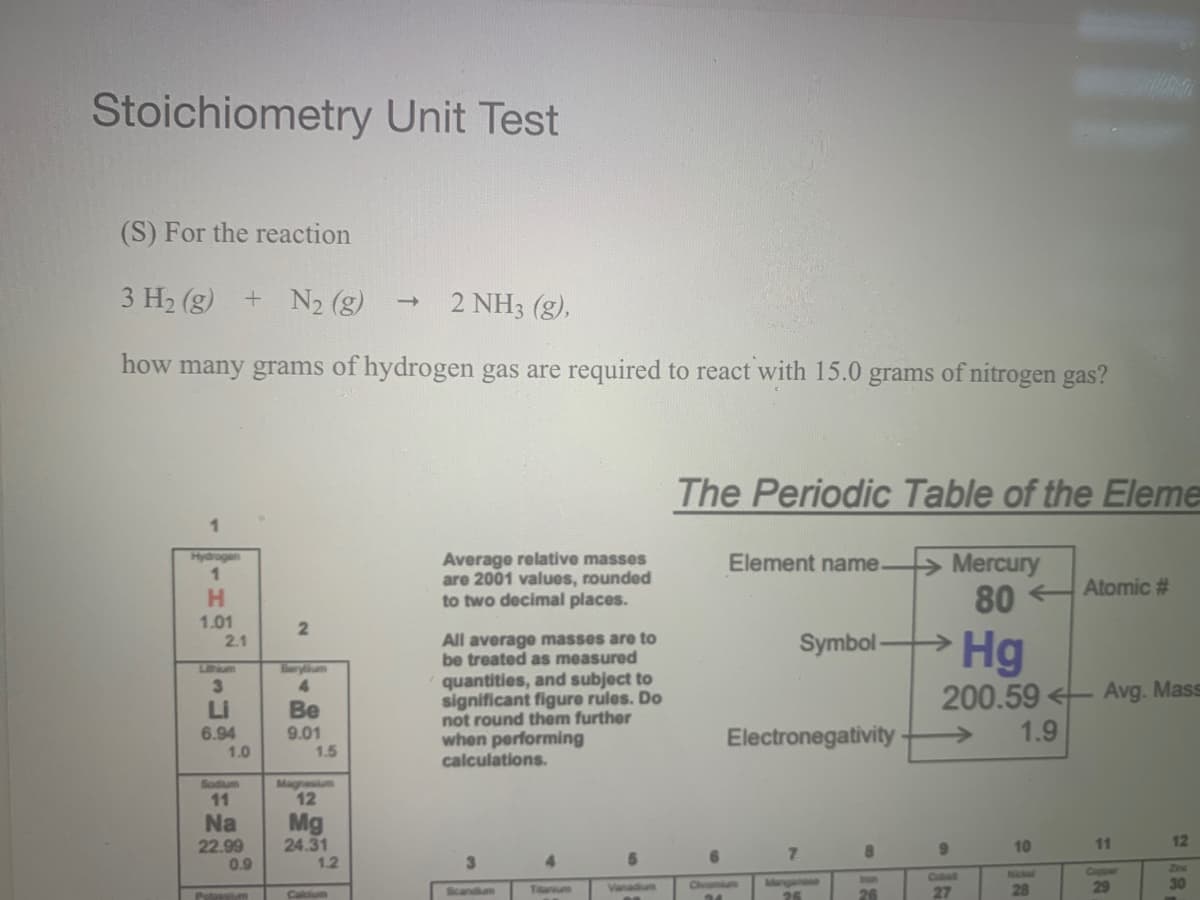
Transcribed Image Text:Stoichiometry Unit Test
(S) For the reaction
3 H2 (g) + N2 (g)
2 NH3 (g),
how many grams of hydrogen gas are required to react with 15.0 grams of nitrogen gas?
The Periodic Table of the Eleme
Hydrogen
Average relative masses
are 2001 values, rounded
to two decimal places.
> Mercury
80
Element name.
Atomic #
H.
1.01
2.1
Hg
All average masses are to
be treated as measured
quantities, and subject to
significant figure rules. Do
not round them further
when performing
calculations.
Symbol-
Berylum
4.
LBum
LI
6.94
1.0
Be
9.01
1.5
200.59 < Avg. Mass
1.9
Electronegativity
->
Sodum
11
Magnesum
12
Mg
24.31
Na
11
12
22.99
0.9
10
6.
7.
1.2
Nical
Copper
Cua
Manganese
25
Potassum
Scandium
Tianium
Vanadum
Chromum
26
27
28
29
30
Calium
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning