Safari File Edit View History Bookmarks Window Help 1)) 48% Sun 10:58 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. Learning Goal: To learn to classify acids and bases and to predict the products of neutralization reactions. Acids are substances that ionize to form free H* ions in solution whereas bases are substances that combine with Ht ions. Strong acids and strong bases completely ionize but weak acid and weak bases only partially ionize. A salt is a term for an ionic compound such as NaCl or MgBr2. When an acid and a base are mixed together, a neutralization reaction occurs. The products of an acid-base reaction do not have the chemical characteristics of either the acid or the base that originally reacted. Part A Classify each substance as a strong acid, strong base, weak acid, or weak base. Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help H2SO4 HCI Кон HF СH3СООН НCN HCOOH (CH3)2NH HI NH3 CSOH HCIO4 HBr HNO3 CH3NH2 HNO2 NaOH Ca(OH)2 Ba(OH)2 LIOH Strong acids Weak acids Strong bases Weak bases FEB 23 tv MacBook Pro esc ! 23 & ) 1 3 4 delete 00
Safari File Edit View History Bookmarks Window Help 1)) 48% Sun 10:58 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. Learning Goal: To learn to classify acids and bases and to predict the products of neutralization reactions. Acids are substances that ionize to form free H* ions in solution whereas bases are substances that combine with Ht ions. Strong acids and strong bases completely ionize but weak acid and weak bases only partially ionize. A salt is a term for an ionic compound such as NaCl or MgBr2. When an acid and a base are mixed together, a neutralization reaction occurs. The products of an acid-base reaction do not have the chemical characteristics of either the acid or the base that originally reacted. Part A Classify each substance as a strong acid, strong base, weak acid, or weak base. Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help H2SO4 HCI Кон HF СH3СООН НCN HCOOH (CH3)2NH HI NH3 CSOH HCIO4 HBr HNO3 CH3NH2 HNO2 NaOH Ca(OH)2 Ba(OH)2 LIOH Strong acids Weak acids Strong bases Weak bases FEB 23 tv MacBook Pro esc ! 23 & ) 1 3 4 delete 00
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Safari
File
Edit View History Bookmarks Window Help
1)) 48%
Sun 10:58 AM
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111...
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Acid-Base Reactions
16 of 24
<>
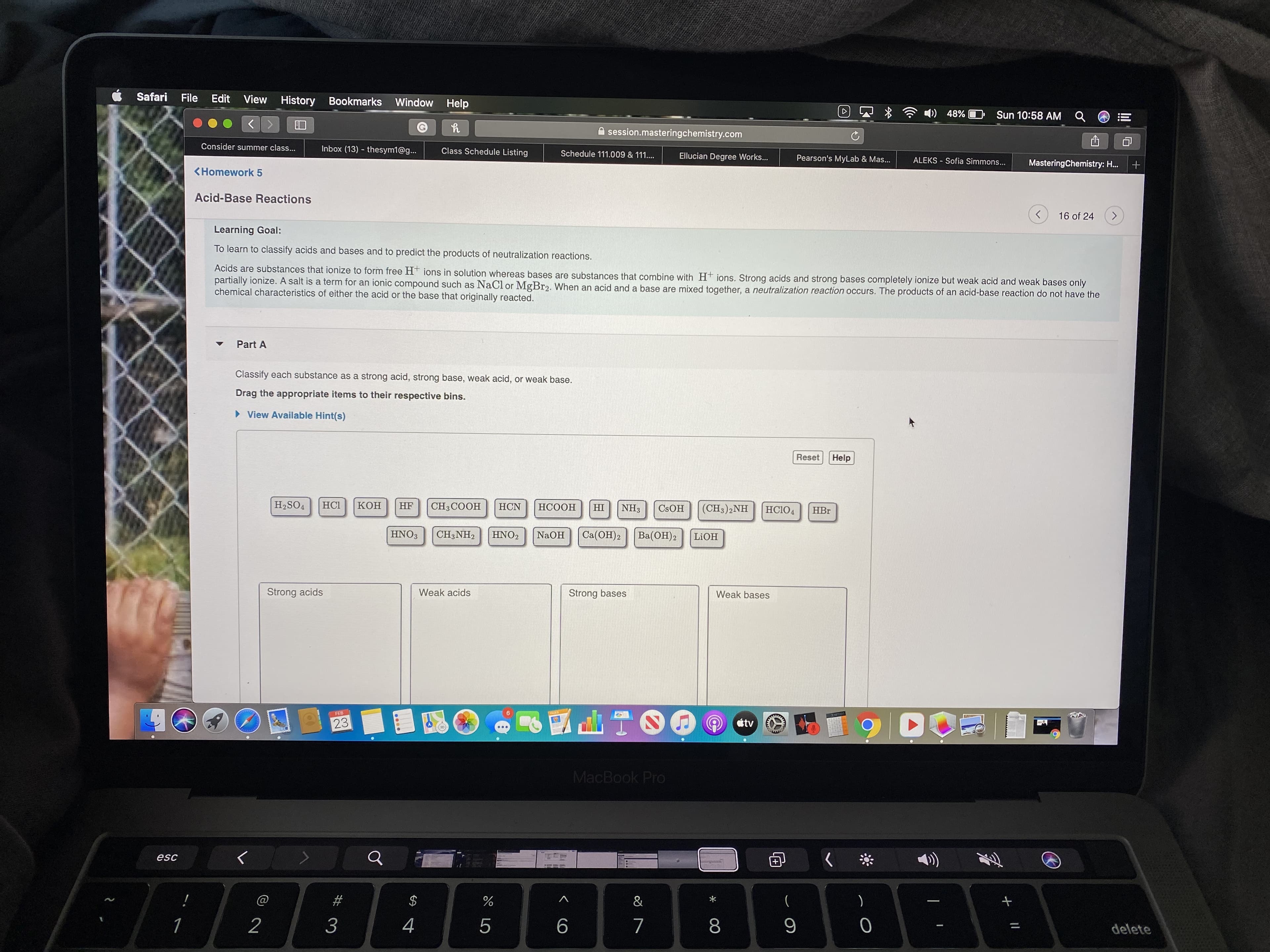
Learning Goal:
To learn to classify acids and bases and to predict the products of neutralization reactions.
Acids are substances that ionize to form free H* ions in solution whereas bases are substances that combine with Ht ions. Strong acids and strong bases completely ionize but weak acid and weak bases only
partially ionize. A salt is a term for an ionic compound such as NaCl or MgBr2. When an acid and a base are mixed together, a neutralization reaction occurs. The products of an acid-base reaction do not have the
chemical characteristics of either the acid or the base that originally reacted.
Part A
Classify each substance as a strong acid, strong base, weak acid, or weak base.
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset
Help
H2SO4
HCI
Кон
HF
СH3СООН
НCN
HCOOH
(CH3)2NH
HI
NH3
CSOH
HCIO4
HBr
HNO3
CH3NH2
HNO2
NaOH
Ca(OH)2
Ba(OH)2
LIOH
Strong acids
Weak acids
Strong bases
Weak bases
FEB
23
tv
MacBook Pro
esc
!
23
&
)
1
3
4
delete
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

