Safari File Edit View History Bookmarks Window Help 50% Sun 10:49 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... If a solute produces ions when dissolved, it is called an electrolyte because the resulting ions in solution will allow the solution to conduct electricity. A solute that completely dissociates into ions is a stronger electrolyte than one that only partially dissociates into ions. If a solute remains as a molecule when dissolved, it is called a nonelectrolyte.In this tutorial, you will practice identifying substances as strong electrolytes, weak electrolytes, or nonelectrolytes. Part A Each of the following reactions shows a solute dissolved in water. Classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. 1. C(1)→C(aq) 2. AB(aq) = A+(aq)+B¯(aq) 3. MN(aq)→M* (aq) + N (aq) 4. XZ(s)→X+(aq) + Z (aq) 5. P(s)→P(aq) Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help AB MN XZ Strong electrolyte Weak electrolyte Nonelectrolyte FEB 6. 3141500 23 átv MacBook Pro esc P. Safari File Edit View History Bookmarks Window Help )) 50% C Sun 10:49 AM Q session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H..
Safari File Edit View History Bookmarks Window Help 50% Sun 10:49 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... If a solute produces ions when dissolved, it is called an electrolyte because the resulting ions in solution will allow the solution to conduct electricity. A solute that completely dissociates into ions is a stronger electrolyte than one that only partially dissociates into ions. If a solute remains as a molecule when dissolved, it is called a nonelectrolyte.In this tutorial, you will practice identifying substances as strong electrolytes, weak electrolytes, or nonelectrolytes. Part A Each of the following reactions shows a solute dissolved in water. Classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. 1. C(1)→C(aq) 2. AB(aq) = A+(aq)+B¯(aq) 3. MN(aq)→M* (aq) + N (aq) 4. XZ(s)→X+(aq) + Z (aq) 5. P(s)→P(aq) Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help AB MN XZ Strong electrolyte Weak electrolyte Nonelectrolyte FEB 6. 3141500 23 átv MacBook Pro esc P. Safari File Edit View History Bookmarks Window Help )) 50% C Sun 10:49 AM Q session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H..
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Safari
File
Edit View History Bookmarks Window
Help
50%
Sun 10:49 AM
A session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111...
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H...
<Homework 5
Electrolytes
6 of 24
<>
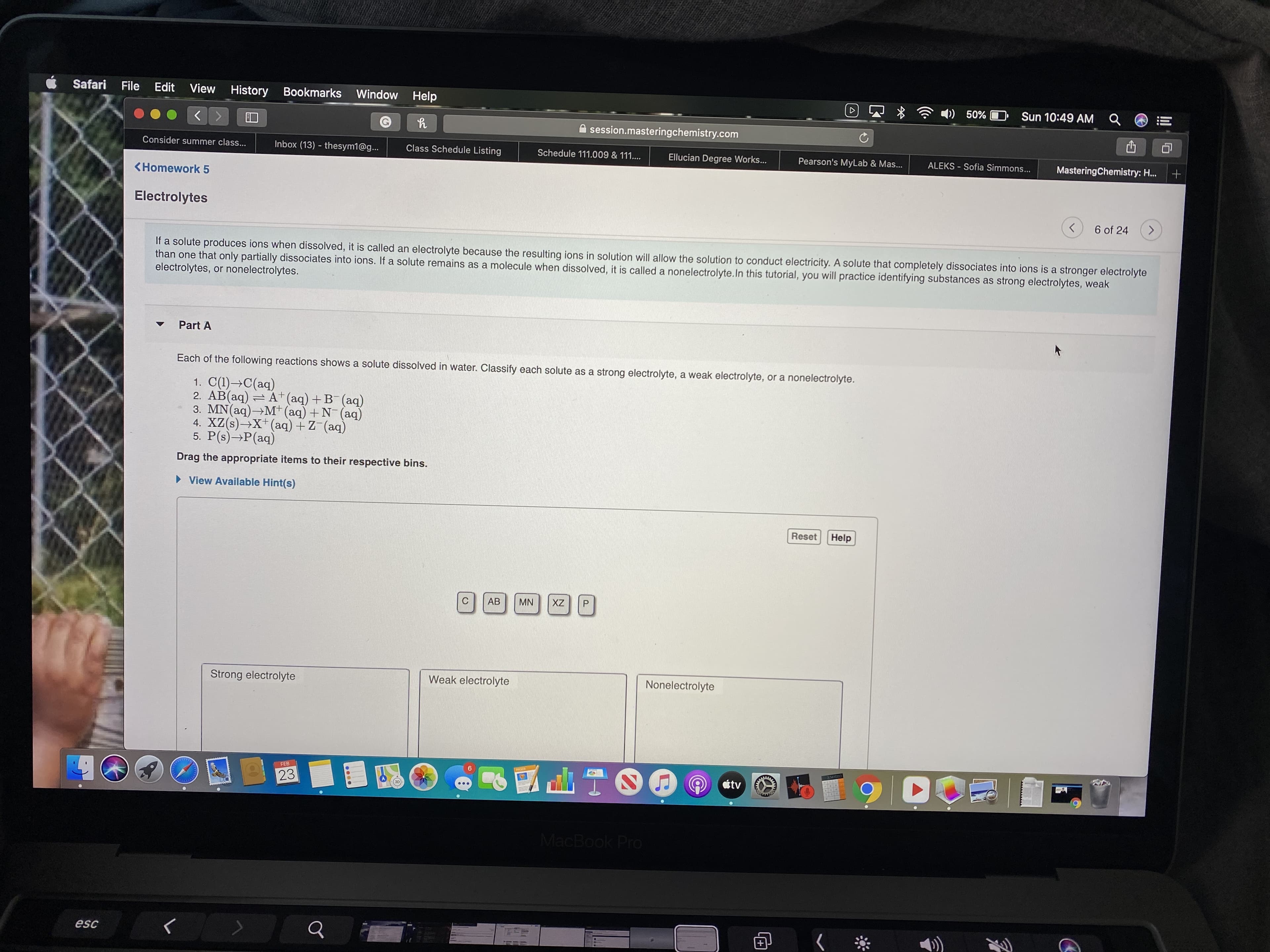
If a solute produces ions when dissolved, it is called an electrolyte because the resulting ions in solution will allow the solution to conduct electricity. A solute that completely dissociates into ions is a stronger electrolyte
than one that only partially dissociates into ions. If a solute remains as a molecule when dissolved, it is called a nonelectrolyte.In this tutorial, you will practice identifying substances as strong electrolytes, weak
electrolytes, or nonelectrolytes.
Part A
Each of the following reactions shows a solute dissolved in water. Classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte.
1. C(1)→C(aq)
2. AB(aq) = A+(aq)+B¯(aq)
3. MN(aq)→M* (aq) + N (aq)
4. XZ(s)→X+(aq) + Z (aq)
5. P(s)→P(aq)
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset
Help
AB
MN
XZ
Strong electrolyte
Weak electrolyte
Nonelectrolyte
FEB
6.
3141500
23
átv
MacBook Pro
esc
P.
Transcribed Image Text:Safari
File
Edit
View History Bookmarks Window Help
)) 50% C
Sun 10:49 AM Q
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111....
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Electrolytes
6 of 24
<.
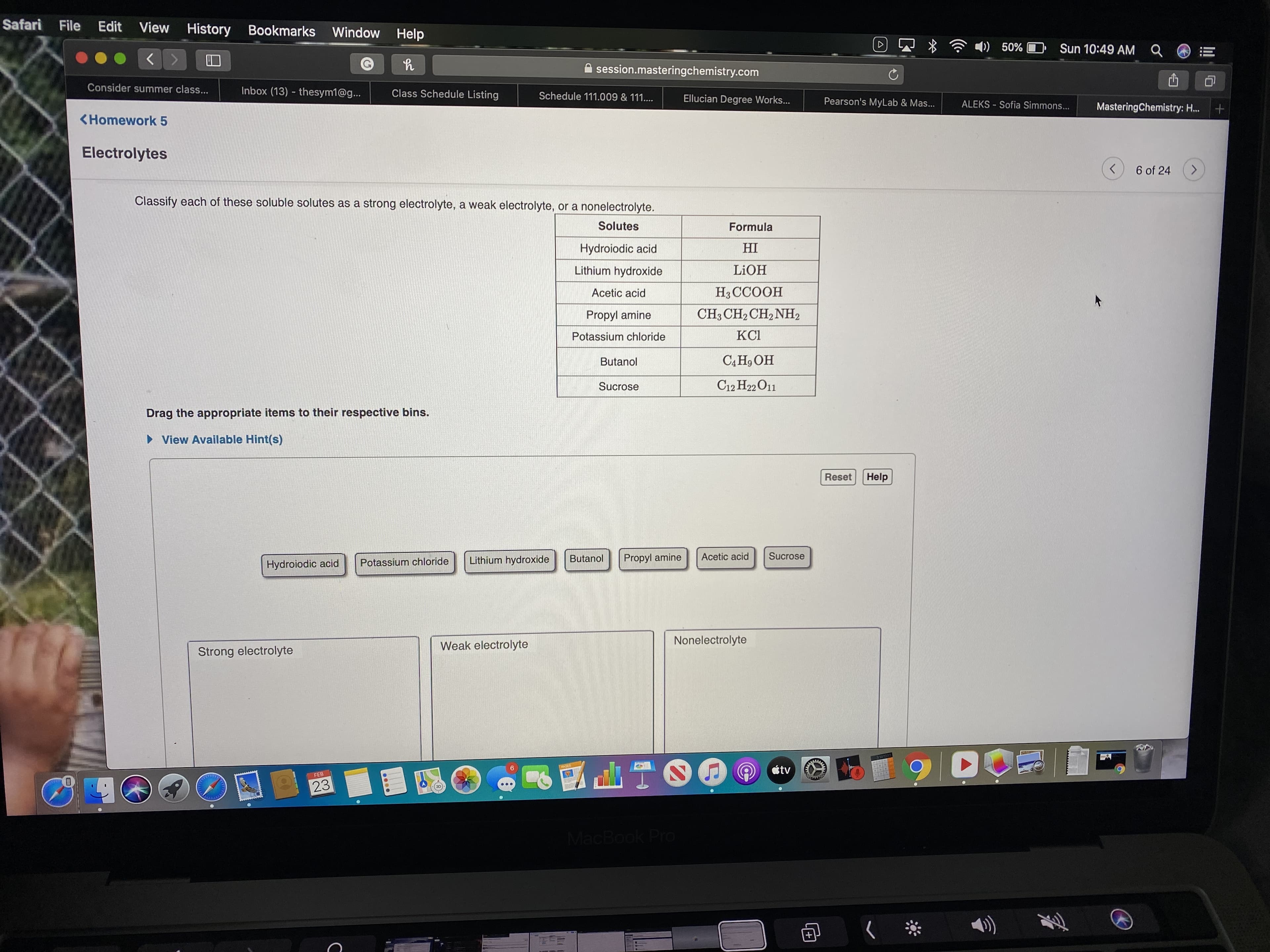
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte.
Solutes
Formula
Hydroiodic acid
HI
Lithium hydroxide
LIOH
Acetic acid
H3CCOOH
Propyl amine
CH3 CH2 CH, NH2
Potassium chloride
KCI
Butanol
СА Но ОН
Sucrose
C12 H22 O11
Drag the appropriate items to their respective bins.
» View Available Hint(s)
Reset
Help
Lithium hydroxide
Butanol
Propyl amine
Acetic acid
Sucrose
Hydroiodic acid
Potassium chloride
Weak electrolyte
Nonelectrolyte
Strong electrolyte
R11159
átv
FEB
23
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

