Samples of hydrated manganese perchlorate are heated to determine the amount of crystal water: Mn(CIO4)2 nH20 -» Mn(CIO4)2 nH20 A sample of 2.126 g weighs 1.473g after heating. The mass of water that evaporated is: The molar ratio of water to manganese perchlorate up to two decimal points is: a.u
Samples of hydrated manganese perchlorate are heated to determine the amount of crystal water: Mn(CIO4)2 nH20 -» Mn(CIO4)2 nH20 A sample of 2.126 g weighs 1.473g after heating. The mass of water that evaporated is: The molar ratio of water to manganese perchlorate up to two decimal points is: a.u
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 39RGQ
Related questions
Question
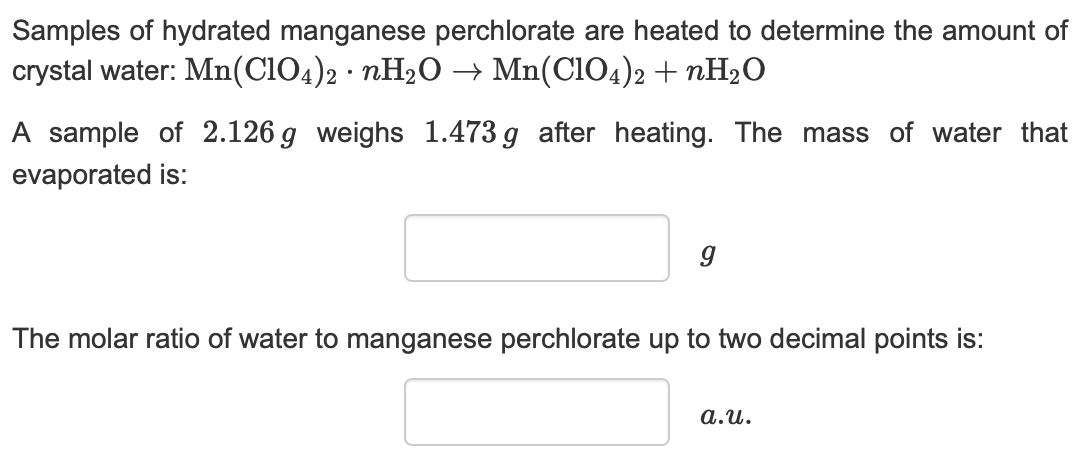
Transcribed Image Text:Samples of hydrated manganese perchlorate are heated to determine the amount of
crystal water: Mn(CIO4)2 nH20 -» Mn(CIO4)2 nH20
A sample of 2.126 g weighs 1.473g after heating. The mass of water that
evaporated is:
The molar ratio of water to manganese perchlorate up to two decimal points is:
a.u
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div