2. Calcium carbonate, when heated strongly in an open test tube, decomposes to yield both the corresponding metal oxide, and a non-metal oxide. The non-metal oxide is a gas, and escapes into the atmosphere. In such a reaction, the following data are collected. Mass of calcium carbonate and test tube before heating 30.46 g Mass of the test tube and contents after heating 27.86 g Mass of empty test tube (a) What type of reaction is this? (b) Write a balanced chemical equation to summarize the reaction. (c) Use the data to illustrate that matter has been conserved, and that to justify the equation that you have written in (b).
2. Calcium carbonate, when heated strongly in an open test tube, decomposes to yield both the corresponding metal oxide, and a non-metal oxide. The non-metal oxide is a gas, and escapes into the atmosphere. In such a reaction, the following data are collected. Mass of calcium carbonate and test tube before heating 30.46 g Mass of the test tube and contents after heating 27.86 g Mass of empty test tube (a) What type of reaction is this? (b) Write a balanced chemical equation to summarize the reaction. (c) Use the data to illustrate that matter has been conserved, and that to justify the equation that you have written in (b).
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 98QAP: Dinitrogen monoxide, N2O, reacts with propane, C3H8, to farm nitrogen, N2; carbon dioxide. CO2; and...
Related questions
Question
be deatailed
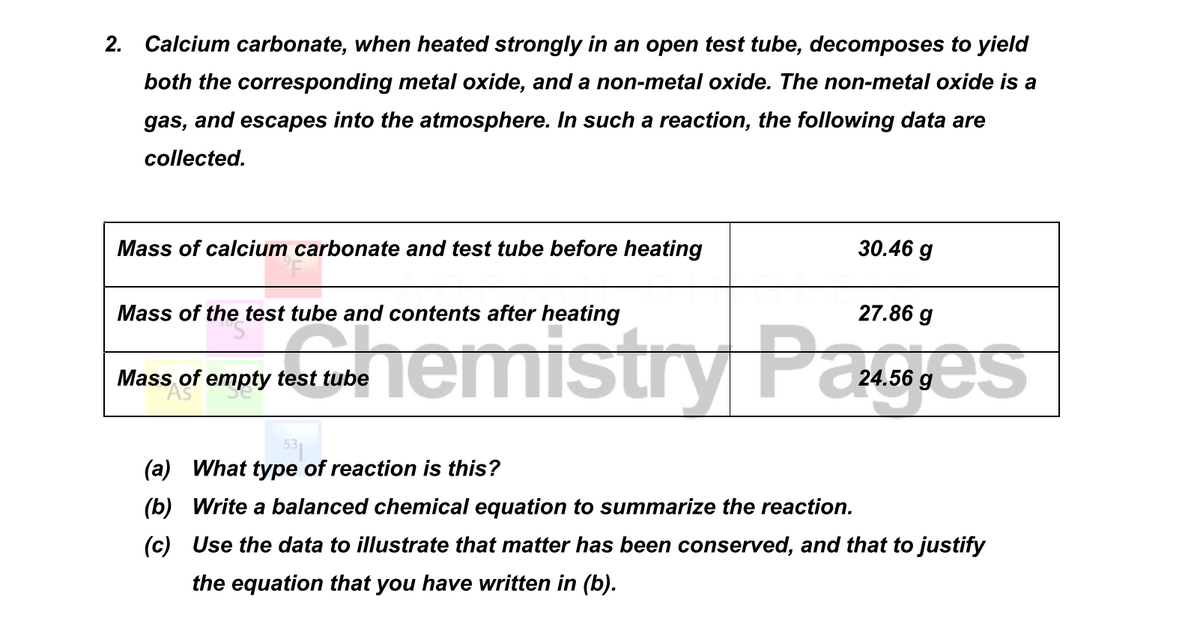
Transcribed Image Text:2. Calcium carbonate, when heated strongly in an open test tube, decomposes to yield
both the corresponding metal oxide, and a non-metal oxide. The non-metal oxide is a
gas, and escapes into the atmosphere. In such a reaction, the following data are
collected.
Mass of calcium carbonate and test tube before heating
30.46 g
Mass of the test tube and contents after heating
27.86 g
hemistry PaGes
Mass of empty test tube
(a) What type of reaction is this?
(b) Write a balanced chemical equation to summarize the reaction.
(c) Use the data to illustrate that matter has been conserved, and that to justify
the equation that you have written in (b).
Expert Solution
Step 1
Given data,
Mass of CaCO3 and test tube before heating = 30.46 g
Mass of contents and test tube after heating = 27.86 g
Mass of vacant test tube = 24.56 g
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning