Limiting reactants Sofia Gaseous butane (CH, (CH,) CH, 2. will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 2.3 g of butane is mixed with 16.1 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to 2 significant digits. do Ar Check Privacy Explanation Terms of Use O 2019 McGraw-Hill Education. All Rights Reserved. 5:09 PM 12/4/2019 99+ O Type here to search hp end home ins prt sc delete fio 144 144 lock - backspace esc ||
Limiting reactants Sofia Gaseous butane (CH, (CH,) CH, 2. will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 2.3 g of butane is mixed with 16.1 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to 2 significant digits. do Ar Check Privacy Explanation Terms of Use O 2019 McGraw-Hill Education. All Rights Reserved. 5:09 PM 12/4/2019 99+ O Type here to search hp end home ins prt sc delete fio 144 144 lock - backspace esc ||
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 114E: a. Write die balanced equation for the combustion of isooctane (C8H18) to produce water vapor and...
Related questions
Question
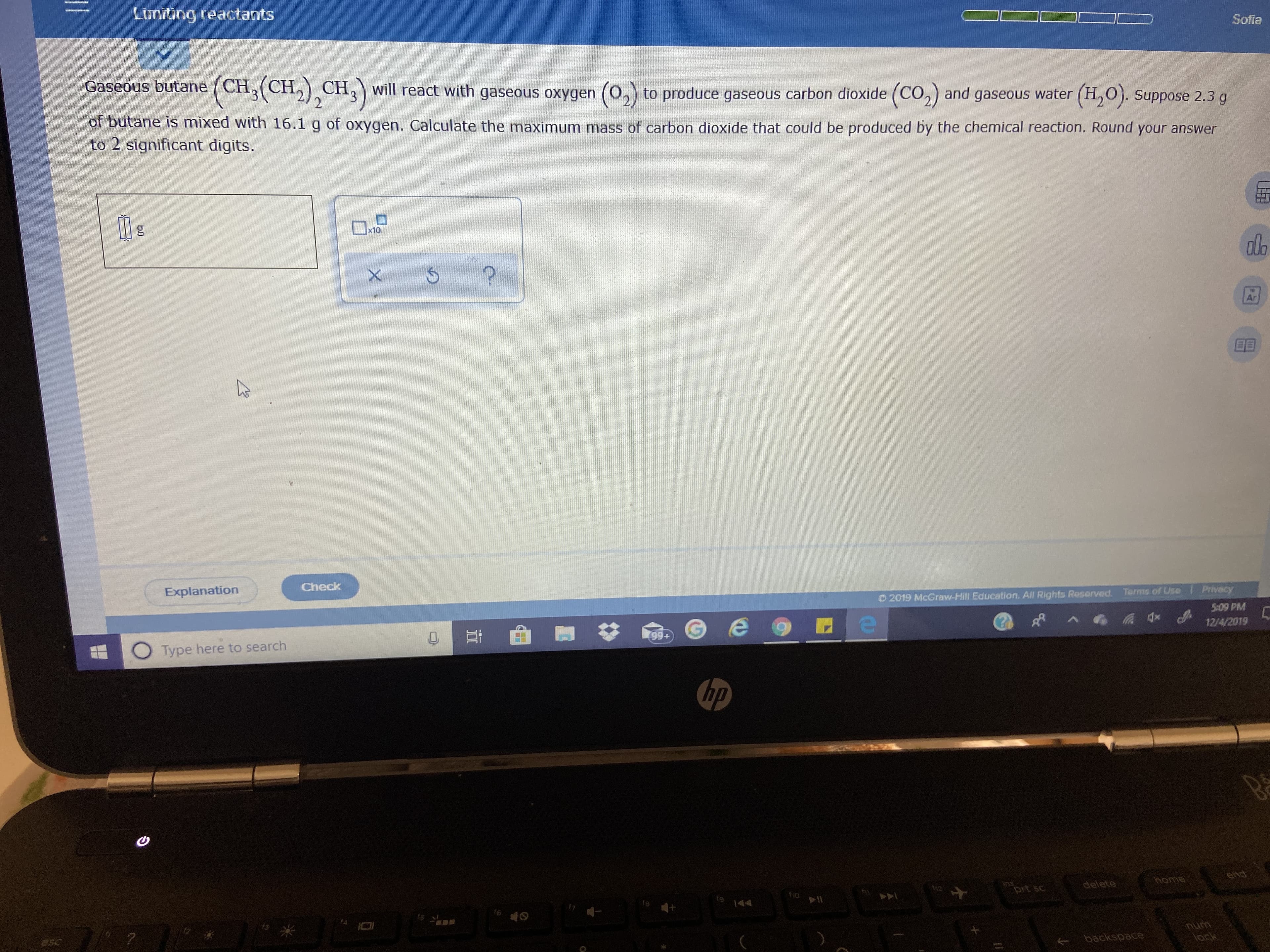
Transcribed Image Text:Limiting reactants
Sofia
Gaseous butane
(CH, (CH,) CH,
2.
will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 2.3 g
of butane is mixed with 16.1 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer
to 2 significant digits.
do
Ar
Check
Privacy
Explanation
Terms of Use
O 2019 McGraw-Hill Education. All Rights Reserved.
5:09 PM
12/4/2019
99+
O Type here to search
hp
end
home
ins
prt sc
delete
fio
144
144
lock
- backspace
esc
||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning