Select all that apply. For the following equilibrium system, which of the following changes will form more CaCO3? CO2(g) + Ca(OH)2(s) = CaCO3(s) + H2O(l) 0 ΔΗ =-113 kJ rxn Decrease temperature at constant pressure (no phase change). Increase volume at constant temperature. Increase partial pressure of CO2. Remove one-half of the initial CaCO3.
Select all that apply. For the following equilibrium system, which of the following changes will form more CaCO3? CO2(g) + Ca(OH)2(s) = CaCO3(s) + H2O(l) 0 ΔΗ =-113 kJ rxn Decrease temperature at constant pressure (no phase change). Increase volume at constant temperature. Increase partial pressure of CO2. Remove one-half of the initial CaCO3.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.95QE: Nitrogen, hydrogen, and ammonia are in equilibrium in a 1000-L reactor at 550 K. The concentration...
Related questions
Question
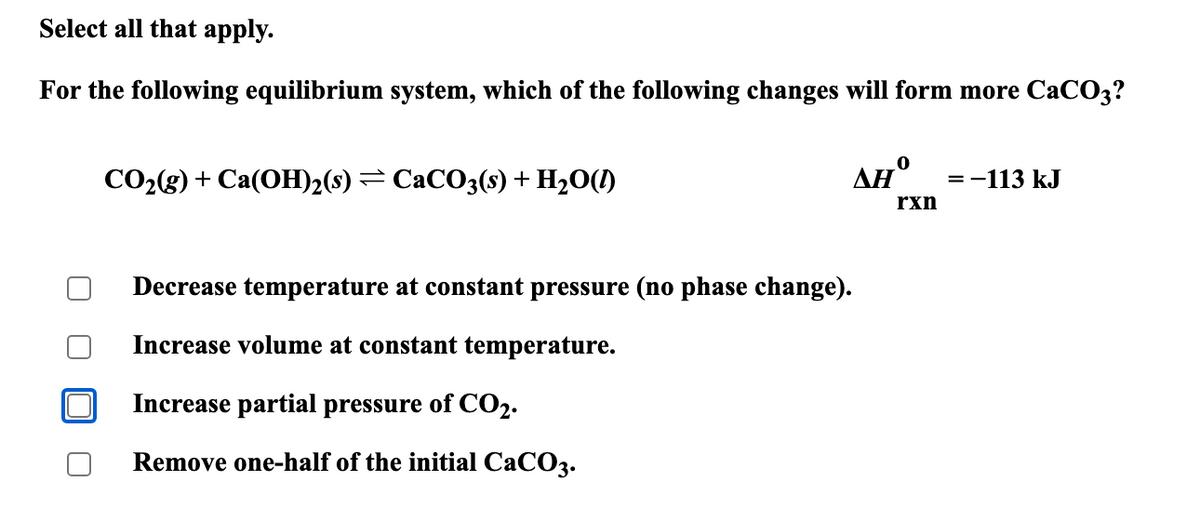
Transcribed Image Text:Select all that apply.
For the following equilibrium system, which of the following changes will form more CaCO3?
CO2(g) + Ca(OH)2(s) = CaCO3(s) + H2O(l)
0
ΔΗ
=-113 kJ
rxn
Decrease temperature at constant pressure (no phase change).
Increase volume at constant temperature.
Increase partial pressure of CO2.
Remove one-half of the initial CaCO3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning