Select the statement(s) that apply to the kinetic molecular theory of gases. Because the space between gas particles is large, gas particles exert no attractive forces on each other. The kinetic energy of gas particles is independent of the temperature. When gas particles collide with each other, they stick together and form a larger particle. A gas consists of particles that move randomly and rapidly The size of gas particles is small compared to the space between the particles When gas particles collide with the wall of a container, they exert no pressure.
Select the statement(s) that apply to the kinetic molecular theory of gases. Because the space between gas particles is large, gas particles exert no attractive forces on each other. The kinetic energy of gas particles is independent of the temperature. When gas particles collide with each other, they stick together and form a larger particle. A gas consists of particles that move randomly and rapidly The size of gas particles is small compared to the space between the particles When gas particles collide with the wall of a container, they exert no pressure.
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
Section: Chapter Questions
Problem 7STP
Related questions
Question
100%
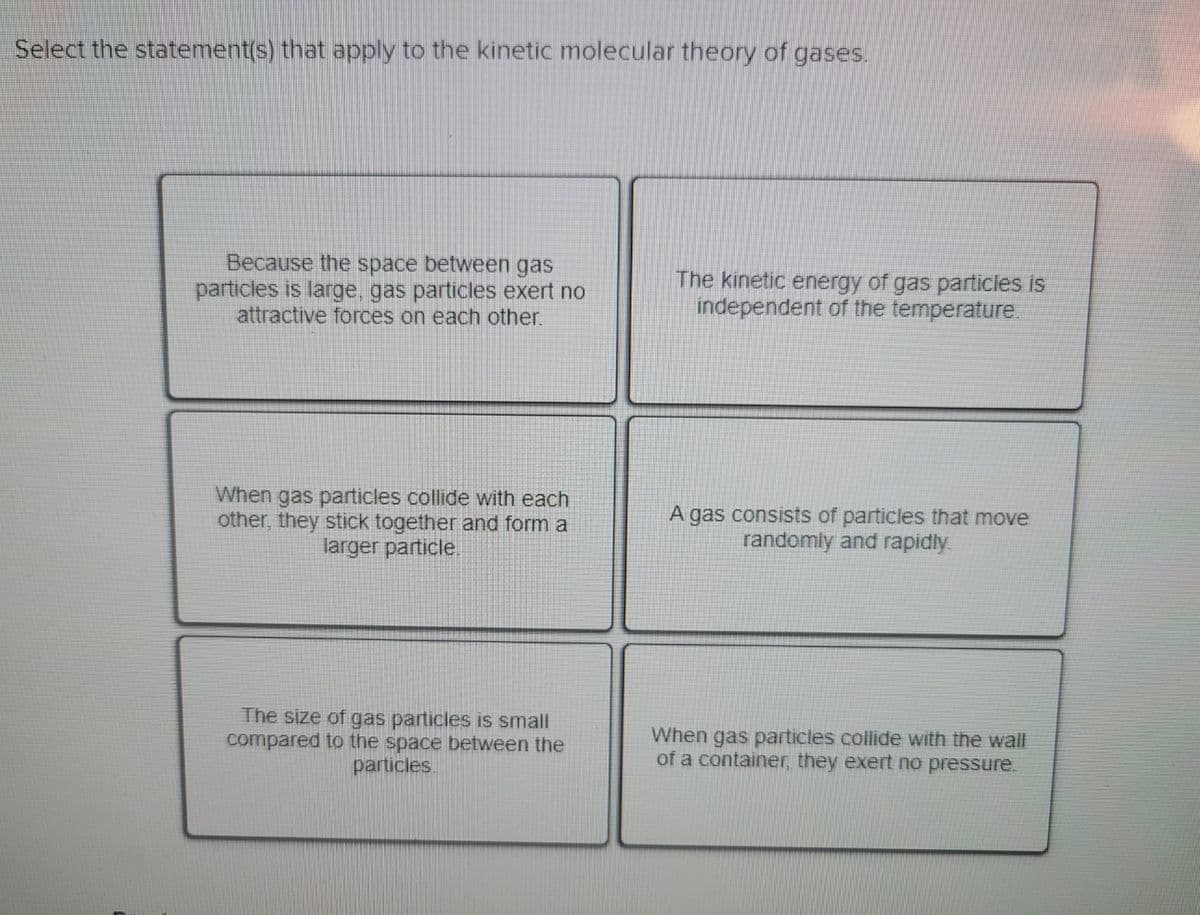
Transcribed Image Text:Select the statement(s) that apply to the kinetic molecular theory of gases.
Because the space between gas
particles is large, gas particles exert no
attractive forces on each other.
The kinetic energy of gas particles is
independent of the temperature.
When gas particles collide with each
other, they stick together and form a
larger particle.
A gas consists of particles that move
randomly and rapidly
The size of gas particles is small
compared to the space between the
particles.
When gas particles collide with the wall
of a container, they exert no pressure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax