Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.3E
Related questions
Question
Show all computations for calories and cal/gm
Formula is already given
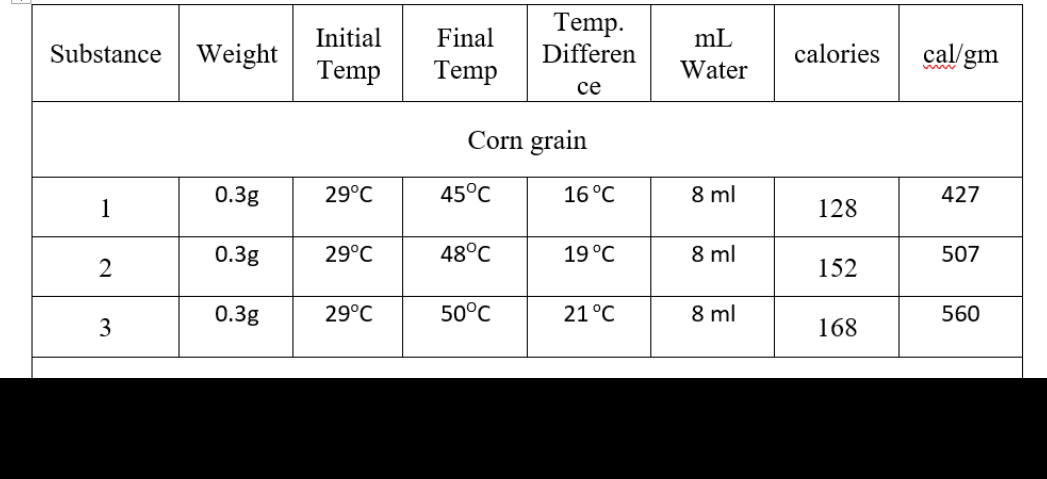
Transcribed Image Text:Temp.
Differen
Initial
Final
mL
Substance
Weight
calories
cal/gm
Temp
Temp
Water
се
Corn grain
0.3g
29°C
45°C
16°C
8 ml
427
1
128
0.3g
29°C
48°C
19 °C
8 ml
507
152
0.3g
29°C
50°C
21°C
8 ml
560
3
168

Transcribed Image Text:Q(Lost by food)=Q(gained by water)
Q(gained by water) = m*c*T
Where,
m- mass of water in grams
c- specific heat capacity of water (1cal/g °C)
T- change in temperature (°C)
ownloaded by 100000829896244 from CourseHero.com on 11-16-2021 08:22:31 GMT -06
.com/file/106239665/act-14docx/
mL to grams
mLx Density of water g/mL
density of water at 30°C is 0.995646
8mL (0995646 g/mL)
= 7.965168g or 8g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning