Show the calculation of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol. Record the values of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol. Ea J/mol Ea kJ/mol
Show the calculation of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol. Record the values of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol. Ea J/mol Ea kJ/mol
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.8QAP
Related questions
Question
Show the calculation of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol.
- Record the values of the Ea for the reaction of S2O82- and I- in J/mol and kJ/mol.
|
Ea |
J/mol |
|
Ea |
kJ/mol |
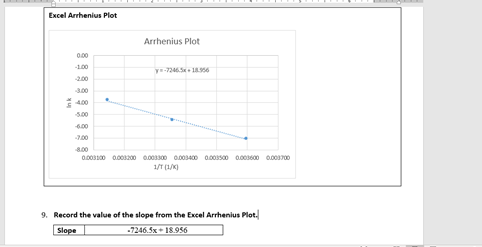
Transcribed Image Text:Excel Arrhenius Plot
Arrhenius Plot
0.00
-1.00
2.00
3.00
* 400
500
6.00
100
8.00
0.003100 0.003200 0.003300 0.003400 0.003sa0 0.003600 0003700
1/T (1/K)
9. Record the value of the slope from the Excel Arrhenius Plot.
Slope
-7246.5x+ 18.956
![Highlight, copy, and paste the Excel Arrhenius Plot for the S20 and I reaction into your lab report where
indicated. The plot should resemble the example shown above. At minimum the plot, must have the following
features:
1. A Title = Arrhenius Plot
2. Axis Labels: y-axis = In k and x-axis = 1/T (1/K)
3. Trendline (see dotted line above) and Trendline Equation
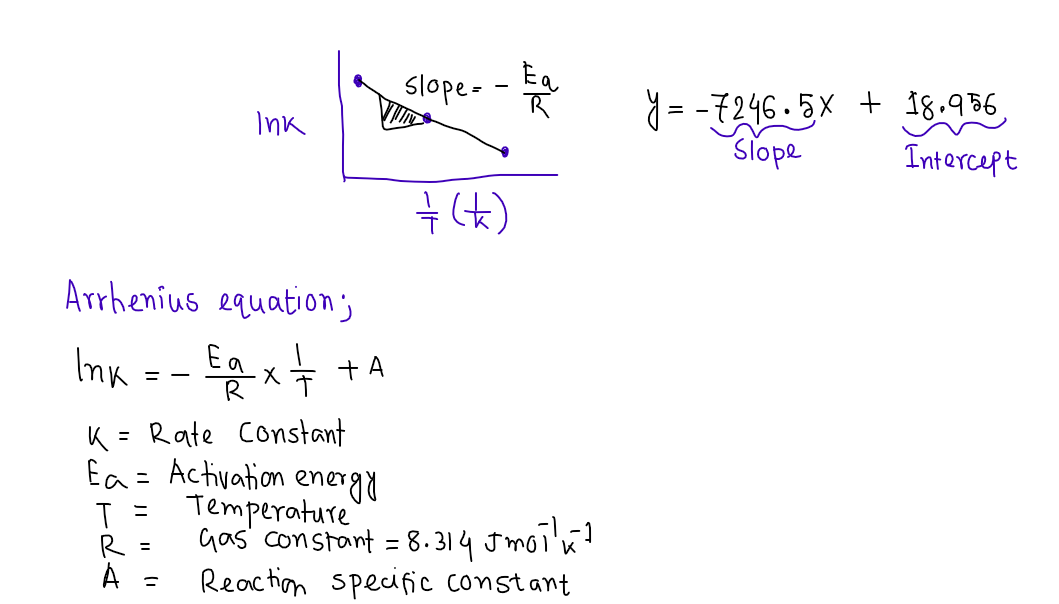
Calculation - Energy of Activation (E,)
Using the slope from the Arrhenius plot, R [8.31 J/(mol-K)], and the equation below, calculate the value of
the E, for the reaction of S;Og* and I in J/mol and kl/mol.
E, = -(R)(slope)
Show your work for the calculation of E, (where indicated) in your lab report.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F23029c3f-32e4-4946-9f07-7f9c026ab9f8%2F5071780f-2099-4675-a871-7c3fad142900%2Frbl6f64_processed.png&w=3840&q=75)
Transcribed Image Text:Highlight, copy, and paste the Excel Arrhenius Plot for the S20 and I reaction into your lab report where
indicated. The plot should resemble the example shown above. At minimum the plot, must have the following
features:
1. A Title = Arrhenius Plot
2. Axis Labels: y-axis = In k and x-axis = 1/T (1/K)
3. Trendline (see dotted line above) and Trendline Equation
Calculation - Energy of Activation (E,)
Using the slope from the Arrhenius plot, R [8.31 J/(mol-K)], and the equation below, calculate the value of
the E, for the reaction of S;Og* and I in J/mol and kl/mol.
E, = -(R)(slope)
Show your work for the calculation of E, (where indicated) in your lab report.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER