Show the calculation of the volume of H₂O gas formed by the combustion of 18.6 grams of C6H6 at 30°C and 1.10 atm? The combustion of benzene (C6H₁) takes place by the following reaction equation. 2 C6H6 (g) + 15 O2 (g) 12 CO2 (g) + 6 H₂O (g)
Show the calculation of the volume of H₂O gas formed by the combustion of 18.6 grams of C6H6 at 30°C and 1.10 atm? The combustion of benzene (C6H₁) takes place by the following reaction equation. 2 C6H6 (g) + 15 O2 (g) 12 CO2 (g) + 6 H₂O (g)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter5: Atomic Theory : The Nuclear Model Of The Atom
Section: Chapter Questions
Problem 50E
Related questions
Question
Please send me the question in 20 minutes it's very urgent plz
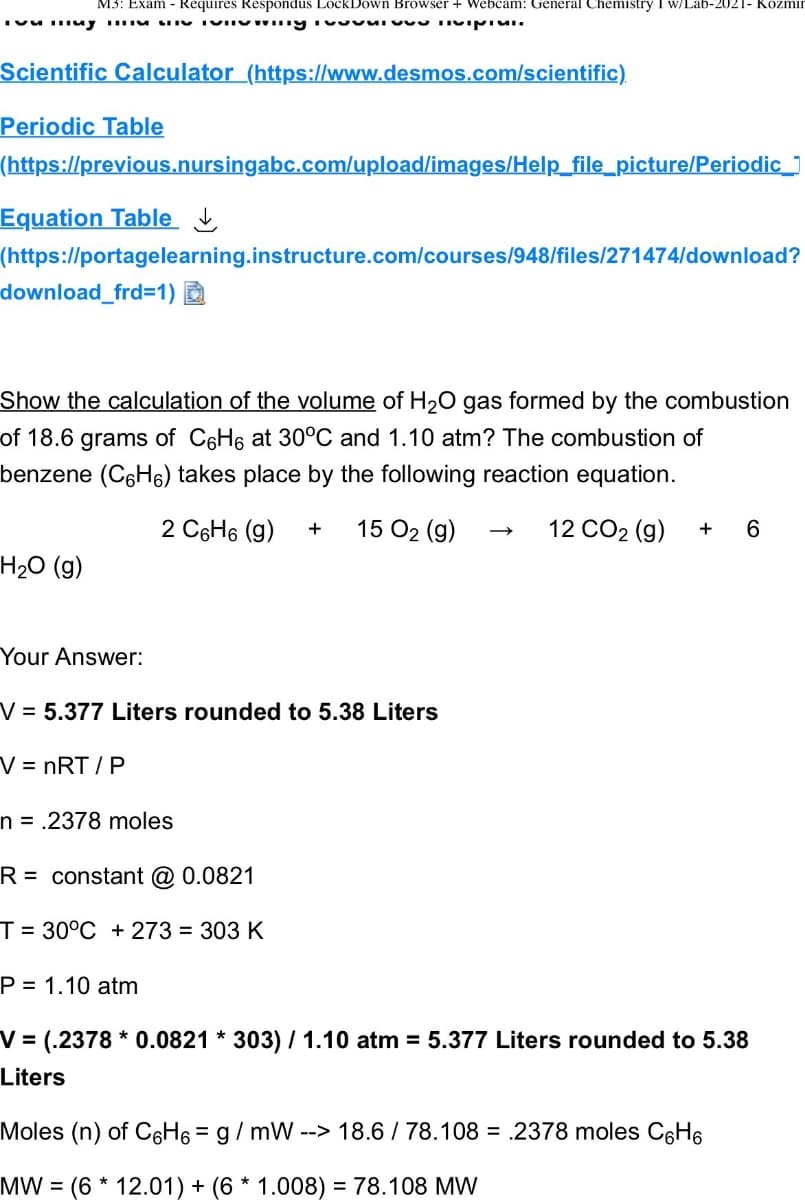
Transcribed Image Text:M3: Exam - Requires Respondus LockDown Browser + Webcam: General Chemistry I w/Lab-2021- Kozmin
muy viving ivvivu Tivipici.
Scientific Calculator (https://www.desmos.com/scientific)
Periodic Table
(https://previous.nursingabc.com/upload/images/Help_file_picture/Periodic_
Equation Table
(https://portagelearning.instructure.com/courses/948/files/271474/download?
download_frd=1)
Show the calculation of the volume of H₂O gas formed by the combustion
of 18.6 grams of C6H6 at 30°C and 1.10 atm? The combustion of
benzene (CH) takes place by the following reaction equation.
2 C6H₁ (9) + 15 0₂ (9)
12 CO2 (g) + 6
H₂O (g)
Your Answer:
V = 5.377 Liters rounded to 5.38 Liters
V = nRT/P
n = .2378 moles
R= constant @ 0.0821
T= 30°C +273 = 303 K
P= 1.10 atm
V = (.2378* 0.0821 * 303) / 1.10 atm = 5.377 Liters rounded to 5.38
Liters
Moles (n) of C6H6 = g/mW --> 18.6 / 78.108 = 2378 moles C6H₁
MW (6 * 12.01) + (6 * 1.008) = 78.108 MW
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
