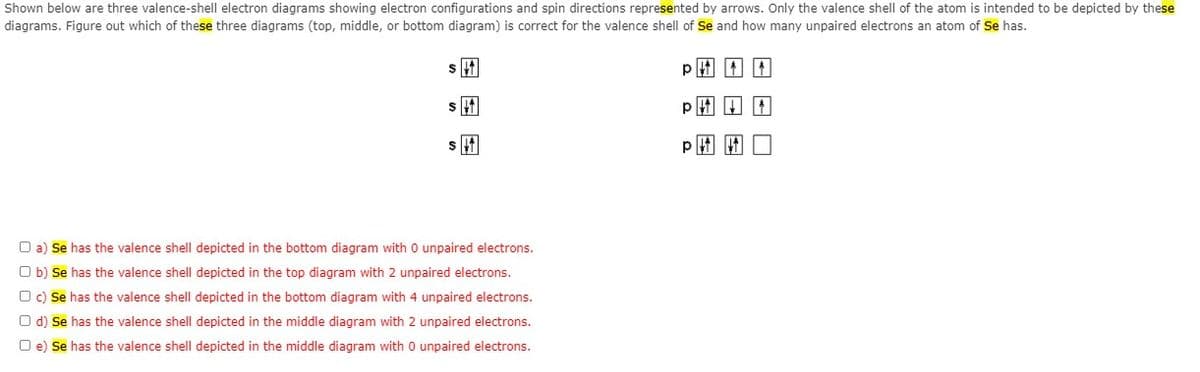
Shown below are three valence-shell electron diagrams showing electron configurations and spin directions represented by arrows. Only the valence shell of the atom is intended to be depicted by these diagrams. Figure out which of these three diagrams (top, middle, or bottom diagram) is correct for the valence shell of Se and how many unpaired electrons an atom of Se has. p田田厂 O a) Se has the valence shell depicted in the bottom diagram with O unpaired electrons. O b) Se has the valence shell depicted in the top diagram with 2 unpaired electrons. O c) Se has the valence shell depicted in the bottom diagram with 4 unpaired electrons. O d) Se has the valence shell depicted in the middle diagram with 2 unpaired electrons. O e) Se has the valence shell depicted in the middle diagram with 0 unpaired electrons.
Shown below are three valence-shell electron diagrams showing electron configurations and spin directions represented by arrows. Only the valence shell of the atom is intended to be depicted by these diagrams. Figure out which of these three diagrams (top, middle, or bottom diagram) is correct for the valence shell of Se and how many unpaired electrons an atom of Se has.
a) Se has the valence shell depicted in the bottom diagram with 0 unpaired electrons.
b) Se has the valence shell depicted in the top diagram with 2 unpaired electrons.
c) Se has the valence shell depicted in the bottom diagram with 4 unpaired electrons.
d) Se has the valence shell depicted in the middle diagram with 2 unpaired electrons.
e) Se has the valence shell depicted in the middle diagram with 0 unpaired electrons.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps









