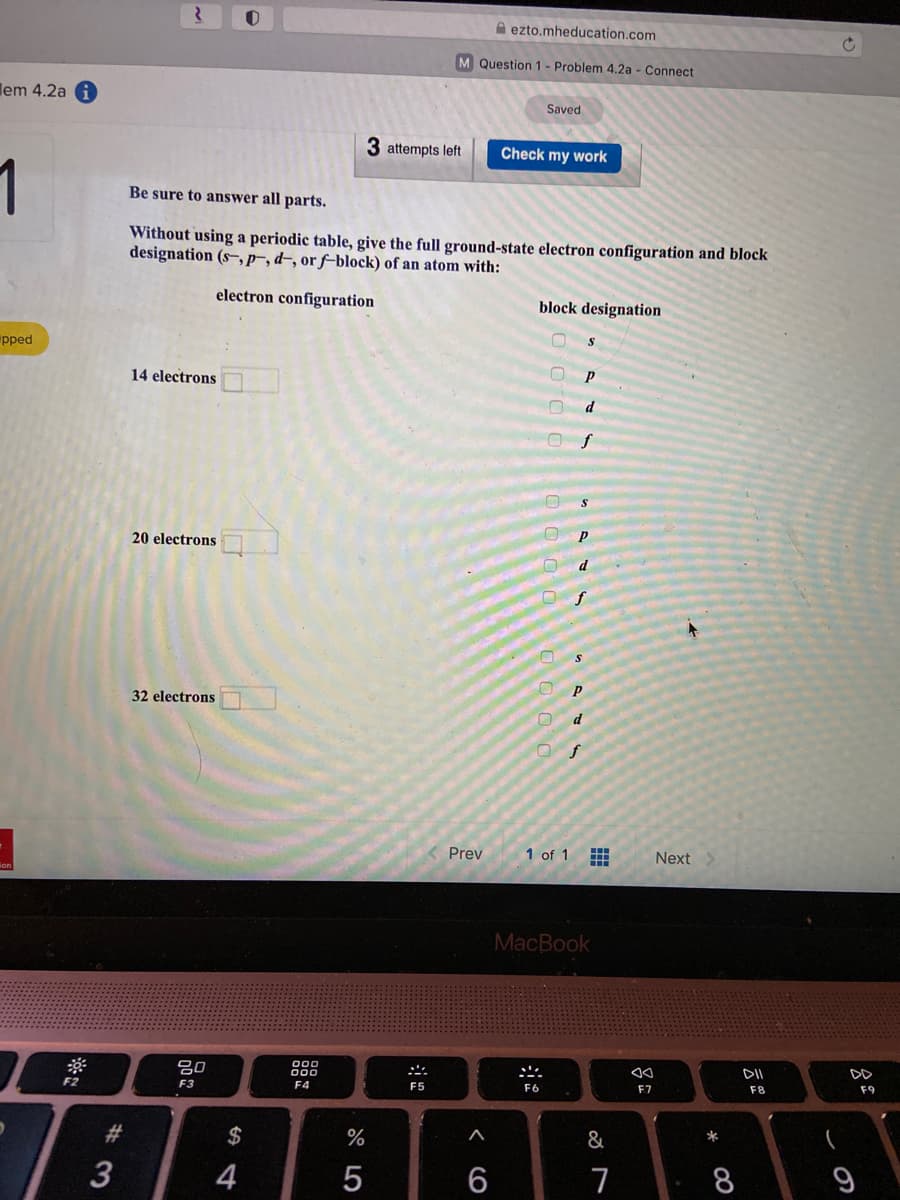
Without using a periodic table, give the full ground-state electron configuration and block designation (s-r,d-, or f-block) of an atom with: electron configuration block designation P 14 electrons d P 20 electrons f 32 electrons d O OO 0 O O O O O O O O
Without using a periodic table, give the full ground-state electron configuration and block designation (s-r,d-, or f-block) of an atom with: electron configuration block designation P 14 electrons d P 20 electrons f 32 electrons d O OO 0 O O O O O O O O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 139QRT
Related questions
Question
Transcribed Image Text:A ezto.mheducation.com
M Question 1 - Problem 4.2a - Connect
lem 4.2a i
Saved
3 attempts left
Check my work
1
Be sure to answer all parts.
Without using a periodic table, give the full ground-state electron configuration and block
designation (s,p-,d-, or f–block) of an atom with:
electron configuration
block designation
pped
14 electrons
d
20 electrons
d
32 electrons
P
f
Prev
1 of 1
Next
MacBook
000
DII
F2
F3
F4
F5
F6
F7
F8
F9
23
2$
%
&
*
3
4
5
6
7
8
9
Expert Solution
Introduction
The electrons are filling in an atom according to the Affbau principle.
The lowest energy level is filled first.
The block in which the element is included is decided by the shell where the valence electrons are filled.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning