Shown below is a concentration vs. time plot for the reaction A = B. For this reaction the value of the equilibrium constant is 0.100 0.080 A E 0.060 0.040 0.020 0.000 0.00 1.00 2.00 3.00 4.00 5.00 Concentration (M)
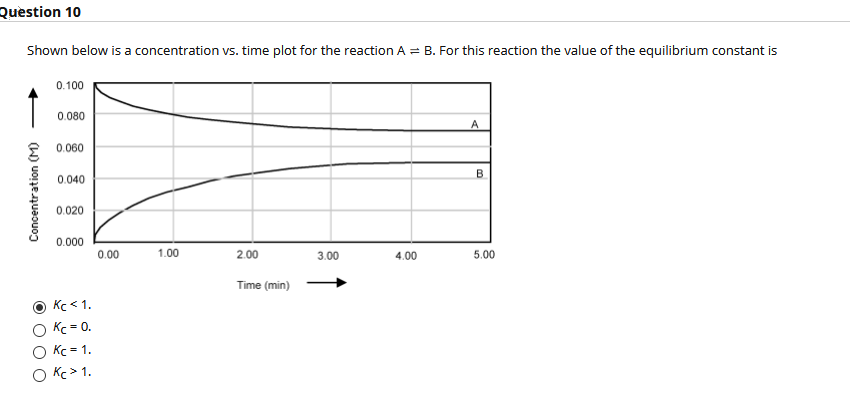
Equilibrium constant is the ratio of concentration of products to the concentration of reactants raised to the power of their respective coefficients.
For example;
Thus,
Here, [ ] denotes the concentration of each specie and a, b are the coefficients of X and Y, respectively.
At any time, if the concentration of reactant (X) is higher than the concentration of product (Y), then;
At any time, if the concentration of product (Y) is higher than the concentration of reactant (X), then;
At any time, if the concentration of product (Y) is equal to the concentration of reactant (X), then;
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









