Sigma Bonding Ac bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH, 1s orbital What atomic or hybrid orbitals make up the sigma bond between Cl and F in chlorine trifluoride, CIF3 ? orbital on Cl + orbital on F What are the approximate F-CI-F bond angles ? (list all possible) Submit Answer Retry Entire Group 8 more group attempts remaining
Sigma Bonding Ac bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH, 1s orbital What atomic or hybrid orbitals make up the sigma bond between Cl and F in chlorine trifluoride, CIF3 ? orbital on Cl + orbital on F What are the approximate F-CI-F bond angles ? (list all possible) Submit Answer Retry Entire Group 8 more group attempts remaining
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter1: Covalent Bonding And Shapes Of Molecules
Section: Chapter Questions
Problem 1.61P: Using cartoon representations, draw a molecular orbital mixing diagram for a CO bond. In your...
Related questions
Question
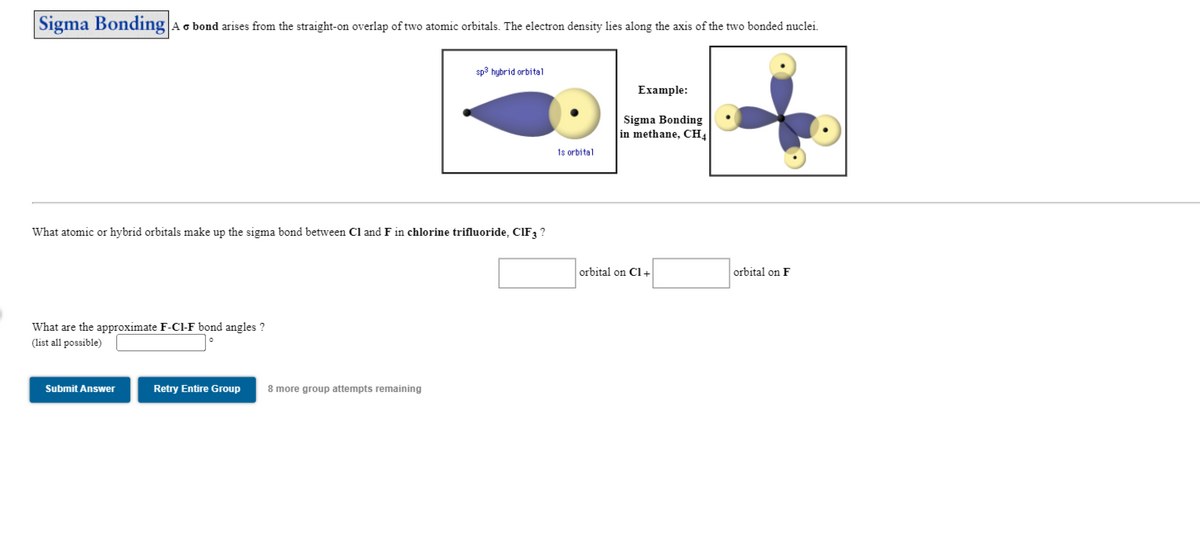
Transcribed Image Text:|Sigma Bonding Ac bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei.
sp3 hybrid orbital
Example:
Sigma Bonding
in methane, CH4
1s orbital
What atomic or hybrid orbitals make up the sigma bond between Cl and F in chlorine trifluoride, CIF ?
orbital on Cl+
orbital on F
What are the approximate F-Cl-F bond angles ?
(list all possible)
Submit Answer
Retry Entire Group
8 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning