Silver arsenate (Ag;AsO4) is a slightly soluble salt having a solubility product of Kp equilibrium 1.0 x 10-22 at 25°C for the Ag3AsO4(s)23 Ag* (aq) + AsO? (aq) (a) Calculate the molar solubility of silver arsenate in pure water at 25°C. (b) Calculate the molar solubility of silver arsenate in 0.10 M AgNO3.
Silver arsenate (Ag;AsO4) is a slightly soluble salt having a solubility product of Kp equilibrium 1.0 x 10-22 at 25°C for the Ag3AsO4(s)23 Ag* (aq) + AsO? (aq) (a) Calculate the molar solubility of silver arsenate in pure water at 25°C. (b) Calculate the molar solubility of silver arsenate in 0.10 M AgNO3.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter18: Chemical Equilibrium
Section: Chapter Questions
Problem 3E
Related questions
Question
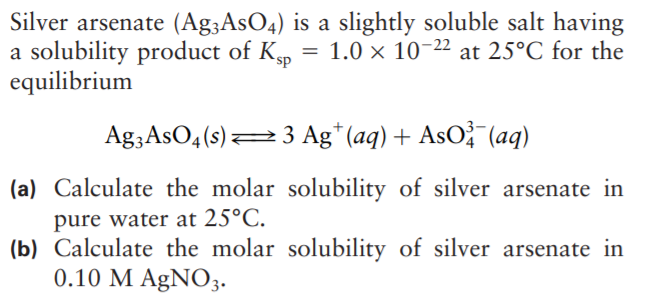
Transcribed Image Text:Silver arsenate (Ag;AsO4) is a slightly soluble salt having
a solubility product of Kp
equilibrium
1.0 x 10-22 at 25°C for the
Ag3AsO4(s)23 Ag* (aq) + AsO? (aq)
(a) Calculate the molar solubility of silver arsenate in
pure water at 25°C.
(b) Calculate the molar solubility of silver arsenate in
0.10 M AgNO3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
