Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH (s) + CO2 (g) → Na2CO3 (s) + H2O () Which is the limiting reactant when 1.85 mol NaOH and 1.00 mol CO2 are allowed to react? How many moles of Na2CO3 can be produced? How many moles of the excess reactant remain after the completion of the reaction?
Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH (s) + CO2 (g) → Na2CO3 (s) + H2O () Which is the limiting reactant when 1.85 mol NaOH and 1.00 mol CO2 are allowed to react? How many moles of Na2CO3 can be produced? How many moles of the excess reactant remain after the completion of the reaction?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.7: Limiting Reactants
Problem 3.18E: Urea is used as a fertilizer because it can react with water to release ammonia, which provides...
Related questions
Question
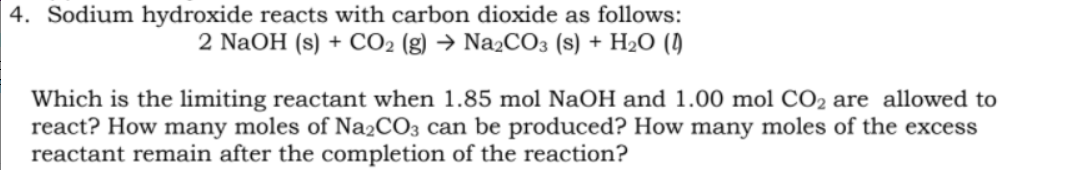
Transcribed Image Text:4. Sodium hydroxide reacts with carbon dioxide as follows:
2 NaOH (s) + CO2 (g) → NażCO3 (s) + H2O (1)
Which is the limiting reactant when 1.85 mol NaOH and 1.00 mol CO2 are allowed to
react? How many moles of Na2CO3 can be produced? How many moles of the excess
reactant remain after the completion of the reaction?

Transcribed Image Text:Direction: Analyzed and solve each problem given on each item. Write your calculation on a
separate sheet of paper.
Note: In calculation involving atomic mass, use 4 significant figure for the atomic mass of each
element needed in the calculation. Use scientific notation, if necessary.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning