Solid aluminum (Al) and oxygen O2) gas react to form solid aluminum oxide (Al203). Suppose you have 1.0 mol of Al and 13.0 mol of O, in a reactor. Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol. l mol x10 ? Submit A Continue Terms of 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro FB F7 O00 OOO F6 F5 F4 & X
Solid aluminum (Al) and oxygen O2) gas react to form solid aluminum oxide (Al203). Suppose you have 1.0 mol of Al and 13.0 mol of O, in a reactor. Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol. l mol x10 ? Submit A Continue Terms of 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro FB F7 O00 OOO F6 F5 F4 & X
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 40PS
Related questions
Question
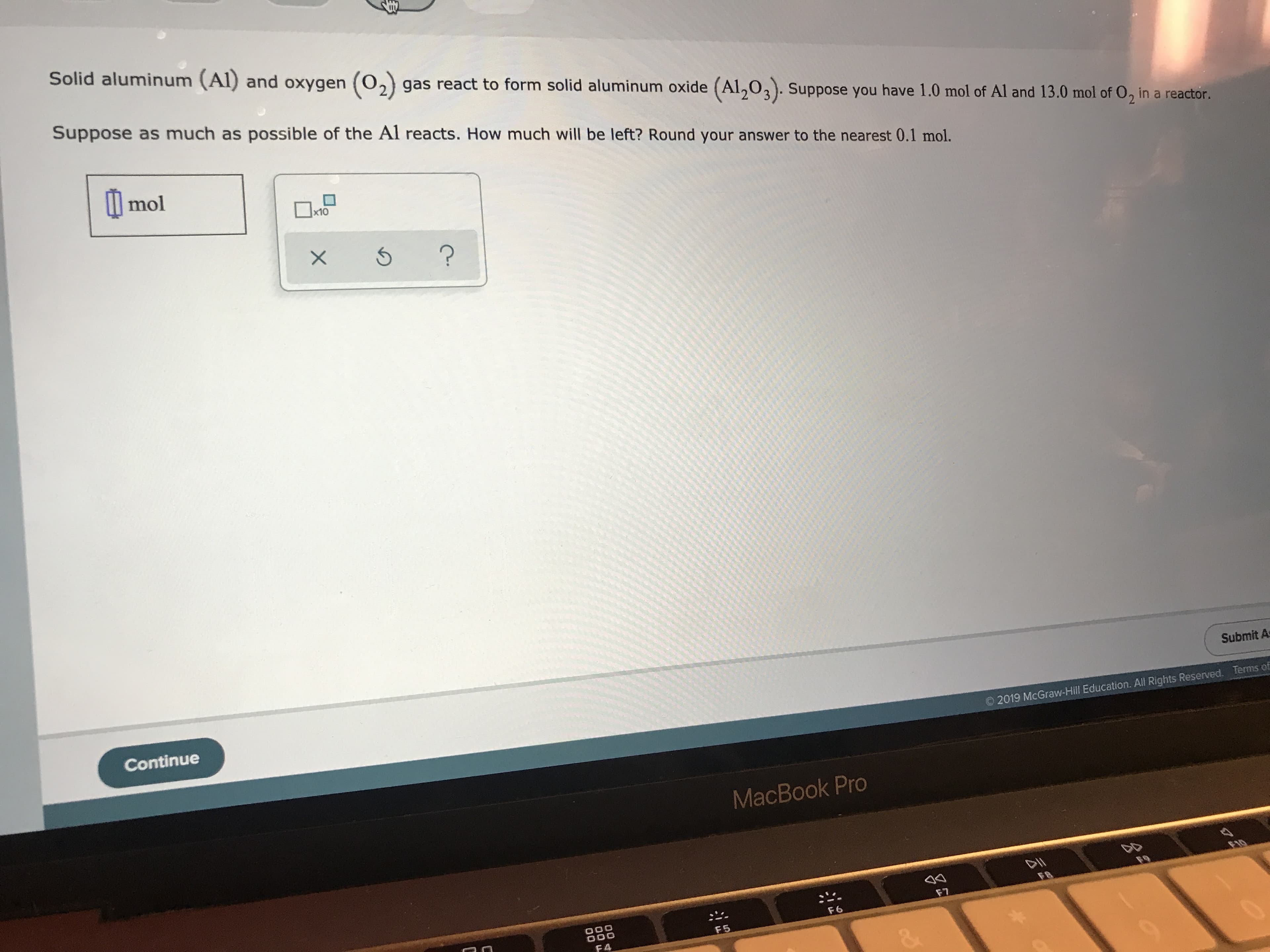
Transcribed Image Text:Solid aluminum (Al) and oxygen O2) gas react to form solid aluminum oxide (Al203). Suppose you have 1.0 mol of Al and 13.0 mol of O, in a reactor.
Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol.
l mol
x10
?
Submit A
Continue
Terms of
2019 McGraw-Hill Education. All Rights Reserved.
MacBook Pro
FB
F7
O00
OOO
F6
F5
F4
&
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
