Solid carbon can react with gaseous water to form carbon mon- oxide gas and hydrogen gas. The equilibrium constant for the reaction at 700.0 Kis K, = 1.60 x 10-3. If a 1.55-L reaction ves- sel initially contains 145 torr of water at 700.0 Kin contact with excess solid carbon, find the percent by mass of hydrogen gas of the gaseous reaction mixture at equilibrium.
Solid carbon can react with gaseous water to form carbon mon- oxide gas and hydrogen gas. The equilibrium constant for the reaction at 700.0 Kis K, = 1.60 x 10-3. If a 1.55-L reaction ves- sel initially contains 145 torr of water at 700.0 Kin contact with excess solid carbon, find the percent by mass of hydrogen gas of the gaseous reaction mixture at equilibrium.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.18PAE: The reaction, 3 H2(g) + N2(g) (g), has the fol lowing equilibrium constants at the temperatures...
Related questions
Question
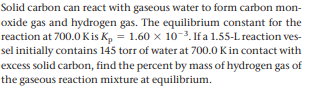
Transcribed Image Text:Solid carbon can react with gaseous water to form carbon mon-
oxide gas and hydrogen gas. The equilibrium constant for the
reaction at 700.0 Kis K, = 1.60 x 10-3. If a 1.55-L reaction ves-
sel initially contains 145 torr of water at 700.0 Kin contact with
excess solid carbon, find the percent by mass of hydrogen gas of
the gaseous reaction mixture at equilibrium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning