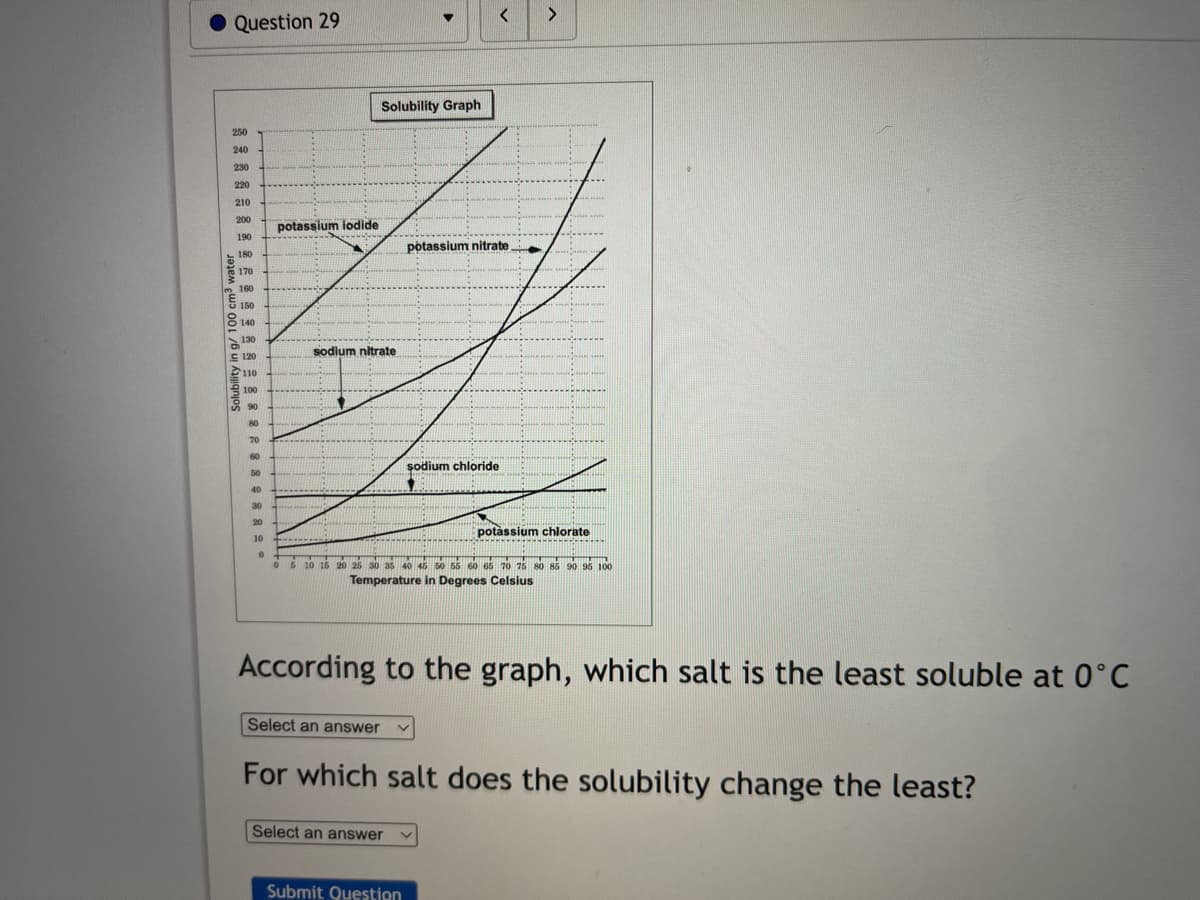
Solubility Graph 250 240 230 220 210 200 potassium iodide 190 potassium nitrate 180 170 m 160 150 8 140 130 sodium nitrate E 120 110 e 100 90 80 70 60 şodium chloride 50 40 30 20 potàssium chlorate 10 ...... 10 16 20 25 30 as 40 45 s0 56 o 65 20 75 so s5 90 96 100 Temperature in Degrees Celsius According to the graph, which salt is the least soluble at 0°C Select an answer For which salt does the solubility change the least? Select an answer
Solubility Graph 250 240 230 220 210 200 potassium iodide 190 potassium nitrate 180 170 m 160 150 8 140 130 sodium nitrate E 120 110 e 100 90 80 70 60 şodium chloride 50 40 30 20 potàssium chlorate 10 ...... 10 16 20 25 30 as 40 45 s0 56 o 65 20 75 so s5 90 96 100 Temperature in Degrees Celsius According to the graph, which salt is the least soluble at 0°C Select an answer For which salt does the solubility change the least? Select an answer
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 83QRT
Related questions
Question
Transcribed Image Text:Question 29
Solubility Graph
250
240
230
220
210
200
potassium iodide
190
potassium nitrate
180
170
160
150
8140
130
sodium nitrate
E 120
110
A 100
8 90
80
70
60
şodium chloride
50
40
30
20
potàssium chlorate
10
5 10 16 20 26 30 35 40 45 50 55 60 66 70 75 80 85 90 95 100
Temperature in Degrees Celsius
According to the graph, which salt is the least soluble at 0°C
Select an answer
For which salt does the solubility change the least?
Select an answer
Submit Question
Solubility in g/ 100 cm3 water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning