Chemicals Amount Benzophenone 0.523 g (2.87*10^-3mol) Sodium borohydride 0.12 g (3.2*10^-3 mol) Benzhydrol 0.357 g (1.94*10^-3mol) Observed melting point 65-66 degree C
Chemicals Amount Benzophenone 0.523 g (2.87*10^-3mol) Sodium borohydride 0.12 g (3.2*10^-3 mol) Benzhydrol 0.357 g (1.94*10^-3mol) Observed melting point 65-66 degree C
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 60QAP: Use Table 17.1 to find Kffor AuCl4- (aq) at 25°C.
Related questions
Question
Chemicals Amount
Benzophenone 0.523 g (2.87*10^-3mol)
Sodium borohydride 0.12 g (3.2*10^-3 mol)
Benzhydrol 0.357 g (1.94*10^-3mol)
Observed melting point 65-66 degree C
Theroretical yield of Benzophenone ________
Percent yield _______________
Theoretical melting point __________--
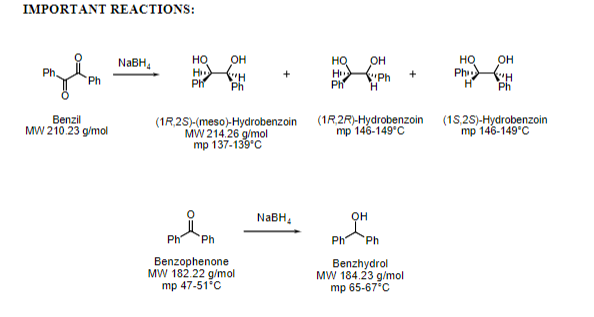
Transcribed Image Text:Ph Ph.
IMPORTANT REACTIONS:
он
H H
Ph
но
он
он
Ph H
Ph
HQ
HQ
H
Ph
NABH,
Ph.
Ph
Benzil
MW 210.23 g/mol
(1R,25)-(meso)-Hydrobenzoin
MW 214.26 g/mol
mp 137-139°С
(1R.2R)-Hydrobenzoin (15,2S)-Hydrobenzoin
mp 146-149°C
mp 146-149°C
NABH,
он
Ph
Ph
Ph
Ph
Benzophenone
MW 182.22 g/mol
mp 47-51°C
Benzhydrol
MW 184.23 g/mol
mp 65-67°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning