Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, 1, and m7. If the value of n= 3 The quantum number I can have values from to The total number of orbitals possible at the n 3 energy level is If the value of / = 2 The quantum umber m, can have values from to The total number of orbitals possible at the /= 2 sublevel is
Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, 1, and m7. If the value of n= 3 The quantum number I can have values from to The total number of orbitals possible at the n 3 energy level is If the value of / = 2 The quantum umber m, can have values from to The total number of orbitals possible at the /= 2 sublevel is
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 135QRT
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
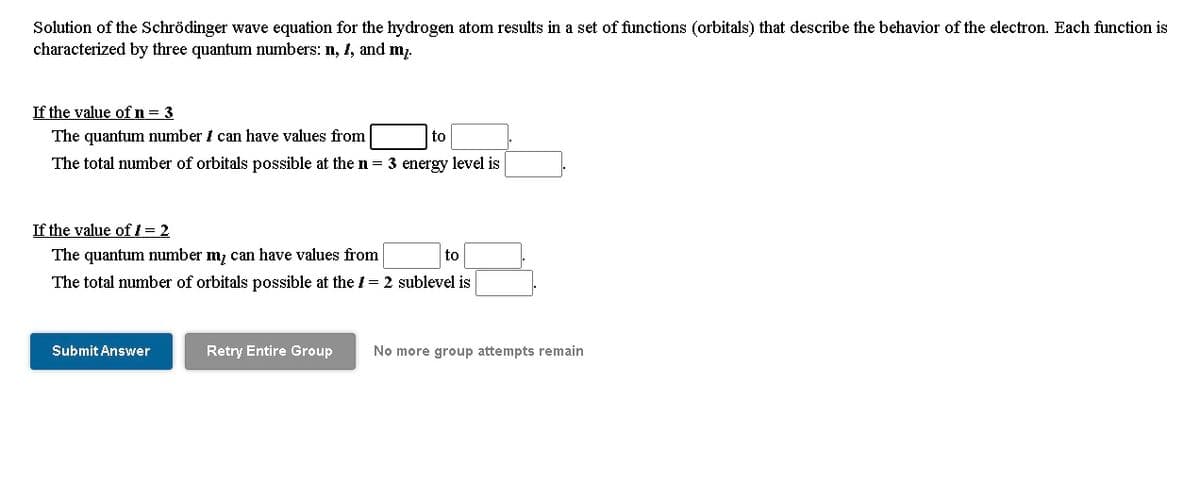
Transcribed Image Text:Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is
characterized by three quantum numbers: n, 1, and m7.
If the value of n= 3
The quantum number i can have values from
to
The total number of orbitals possible at the n = 3 energy level is
If the value of ! = 2
The quantum number m; can have values from
to
The total number of orbitals possible at the /= 2 sublevel is
Submit Answer
Retry Entire Group
No more group attempts remain
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning