Solving applied problems with first-order kinetics Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of X is 2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask with Xand Yand exposes the flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y O yes As both gases decompose, will the partial pressure of X ever fall below the partial pressure of Y? x10 no ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min Check Explanation Privacy 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Insert Home End EsC + F12 F11 F10 F8 F9 F7 F6 F5 F4 Lock F3 F2 F1 X Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to u 2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y As both gases decompose, will the partial pressure of X ever fall below the partial pressure of Y? yes D x10 no ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min Check Explanation O 2019 McGraw-Hill Educat X
Solving applied problems with first-order kinetics Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of X is 2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask with Xand Yand exposes the flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y O yes As both gases decompose, will the partial pressure of X ever fall below the partial pressure of Y? x10 no ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min Check Explanation Privacy 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Insert Home End EsC + F12 F11 F10 F8 F9 F7 F6 F5 F4 Lock F3 F2 F1 X Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to u 2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y As both gases decompose, will the partial pressure of X ever fall below the partial pressure of Y? yes D x10 no ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min Check Explanation O 2019 McGraw-Hill Educat X
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 82GQ: We know that the decomposition of SO2Cl2 is first-order in SO2Cl2, SO2Cl2 SO2(g) + Cl2(g) with a...
Related questions
Question
Calculate time it takes the partial pressure of X too far below the partial pressure of white round your answer to two significant digits if the answer is yes.
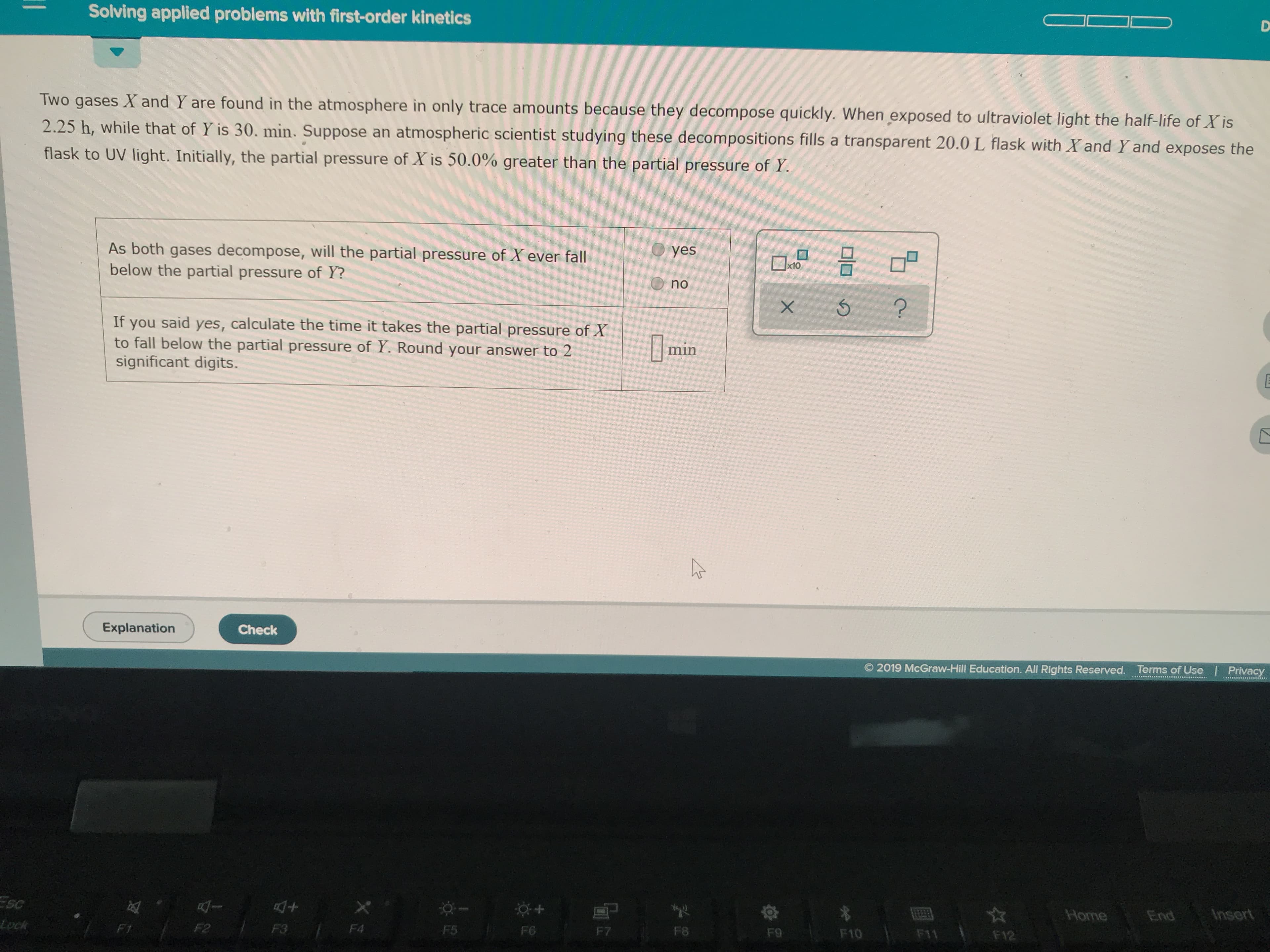
Transcribed Image Text:Solving applied problems with first-order kinetics
Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of X is
2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask with Xand Yand exposes the
flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y
O yes
As both gases decompose, will the partial pressure of X ever fall
below the partial pressure of Y?
x10
no
?
If you said yes, calculate the time it takes the partial pressure of X
to fall below the partial pressure of Y. Round your answer to 2
significant digits.
min
Check
Explanation
Privacy
2019 McGraw-Hill Education. All Rights Reserved. Terms of Use
Insert
Home
End
EsC
+
F12
F11
F10
F8
F9
F7
F6
F5
F4
Lock
F3
F2
F1
X
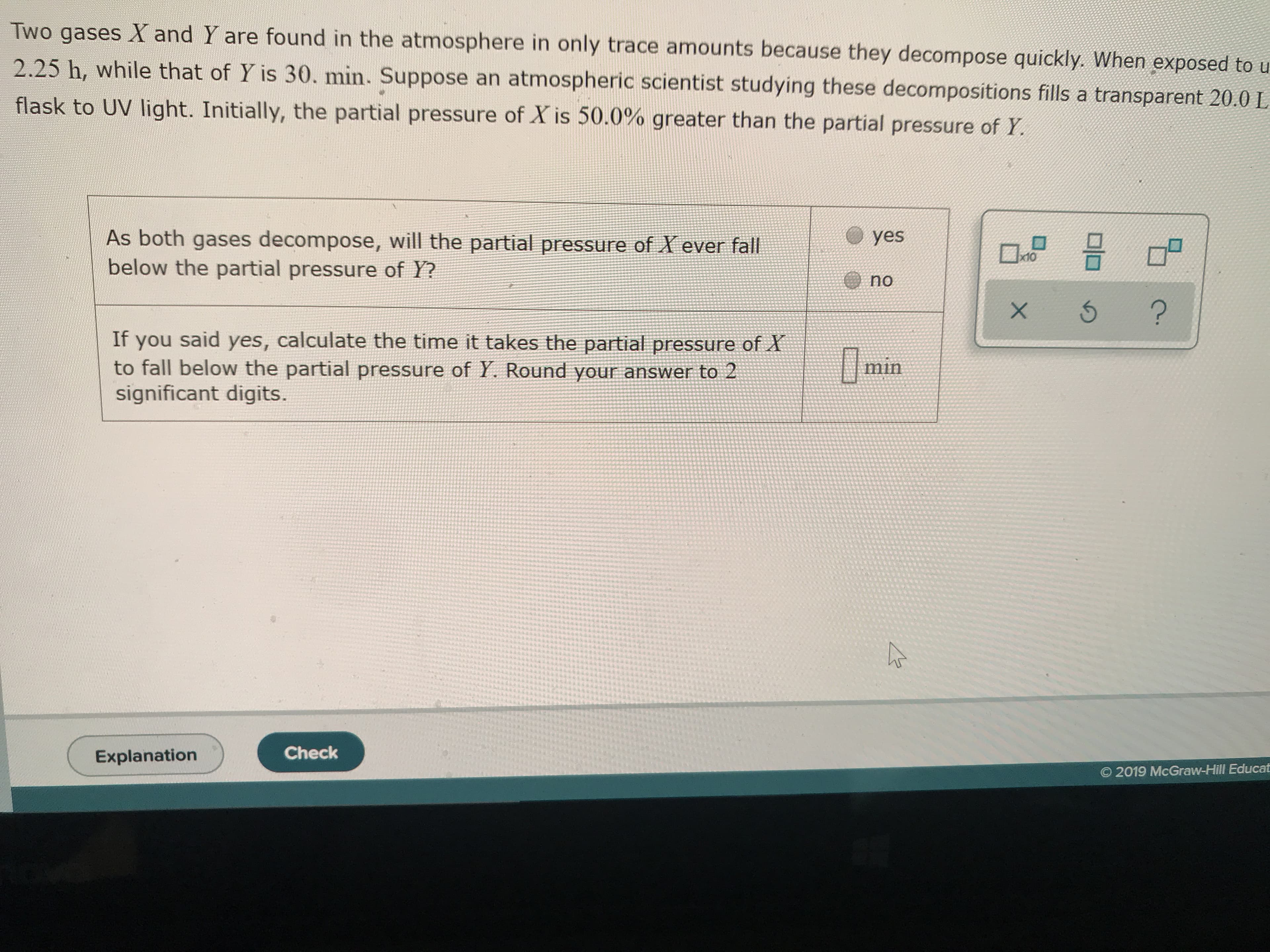
Transcribed Image Text:Two gases X and Y are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to u
2.25 h, while that of Y is 30. min. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L
flask to UV light. Initially, the partial pressure of X is 50.0% greater than the partial pressure of Y
As both gases decompose, will the partial pressure of X ever fall
below the partial pressure of Y?
yes
D
x10
no
?
If you said yes, calculate the time it takes the partial pressure of X
to fall below the partial pressure of Y. Round your answer to 2
significant digits.
min
Check
Explanation
O 2019 McGraw-Hill Educat
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning