Q: What is the oxidation state of each element in COH,? C H -1 What is the oxidation state of each…
A: Oxidation state is also sometimes known as oxidation number. It tells about the degree of oxidation…
Q: Determine the oxidation state for Sodium Iodide, NaI
A: The oxidation state (OS) of an element corresponds to the number of electrons, e-, that an atom…
Q: What is the oxidation number of vanadium in this compound? VO2ClO4
A:
Q: Determine the oxidation state for Gadolinium (III) Chloride, GdCl3
A: Oxidation state of GdCl3: The sum of oxidation numbers for neutral compounds must be 0.
Q: What is the oxidation state of the atoms in bold?
A: Oxidation state of atoms in the bold = ?
Q: Indicate the oxidation state of the underlined element in the following species. Show all pertinent…
A:
Q: Which compound contains nitrogen in a +4 oxidation state? C. NO2 A. N2 B. NH3 D. HNO2
A: In the molecule, the mathematical sum of the each species oxidation state will be equivalent to the…
Q: Determine the highest possible oxidation state for each element.a. V b. Re c. Pd
A: Oxidation state is the total number of electrons lost or gained by an atom when it is chemically…
Q: The metal ions of analytical group IIA are precipitated as Select one: O a. Halides O b. Hydroxides…
A: Metal precipitate depand group reagent of group
Q: Write a balanced chemical equation based on the following description: aqueous iridium(III) bromide…
A: Balanced Equation Based on Description in the Question
Q: Determine the highest possible oxidation state for element. V
A: The term oxidation state is also referred to as oxidation number. It describes the degree of…
Q: provide the correct balanced equation to represent the reaction described. Aluminium bromide…
A: Given -> Reagents are -> Aluminium bromide= AlBr3 Chlorine gas = Cl2
Q: Describe the reaction of aqueous solutions ofsodium sulfide and copper(II) sulfate, producing…
A: The reactions between aqueous solutions of sodium sulfide and copper(II) sulfate forming copper (II)…
Q: 19. Determine the highest possible oxidation state for each element.
A: The term oxidation state is also referred to as oxidation number. It describes the degree of…
Q: For each of the following compounds or ions provide the oxidation state of the metal, dn count and…
A:
Q: What is the oxidation state of bromine in the compound NaBro,? a) +2 b) -3 c) -4 d) +5
A: Correct answer is (d) +5. NaBrO3 +1 + x + ( -2 x 3) = 0 x = +5
Q: Determine the oxidation state of P in PO 3 3-. +6 -3 +3 +2 0
A:
Q: The oxidation state/number for X in XO3- is: +5 -5 -3 +3 0
A:
Q: Fluorine does not exhibit any positive oxidation state. Why?
A: A number of electrons gained or lost by an atom for the formation of chemical bond represented by…
Q: Enter the oxidation state of copper for each form in which it appears in the copper cycle of…
A: Name and the inorganic compounds: (a) CuNO32: It is an inorganic compound which forms blue…
Q: Give the possible oxidation states for each element
A: We have to assign possible oxidation states for given elements from given choices of options
Q: Does the roman numeral refers to the oxidation state of an element?
A: The oxidation state of the metal refers to the number of bonds formed by that element.
Q: does transition elements are able to show variable oxidation states. if yes, why ?
A: The d block elements or elements which have is (n-1) d1–10 ns 1–2 electronic configuration is known…
Q: What is the oxidation state of cobalt (Co) in Col2?
A: The compound given is CoI2 Since there are 7 valence electrons in I. Hence it will take 1 electron…
Q: Fe(CO), エ、エ
A:
Q: The formation of gas bubbles when cobalt reacts with hydrochloric acid.
A: Balance Chemical equation means no of atoms or ions should be equaal in both side reactant and…
Q: A substance that changes color when exposed to acid or base solutions is called a/an ......
A: Titration is the technique of determining the unknown concentration of one solution from the known…
Q: What is the reaction that takes place when a metal is reacting with acids? For example;…
A:
Q: what are the oxidation states of Ag, Zn, Cd and Al
A: To find: The oxidation states of Ag, Zn, Cd, and Al?
Q: Determine the highest possible oxidation state for element Re
A: Oxidation state: Oxidation states are a combination of numbers, positive, and negative signs that…
Q: The oxidation state of the element Au in AuCl4- , Mn in MnO2 is ____and ___ respectively Multiple…
A:
Q: What is the oxidation state of each element in Mn(ClO,),? Cl Mn
A: Molecular formula Mn(ClO4) 3
Q: What is the oxidation state of Cl in each ion? (a) ClO- (b) CIO2- (c) CIO3- (d) CIO4-
A: Question 1 a) ClO- = The oxidation number of oxygen is -2. The oxidation state of Cl in this given…
Q: The oxidation number of Mn in MnO₂ is
A: The oxidation number of an element represents the charge density on the element which controls its…
Q: What is the oxidation state of each element in Mn(NO,),?
A: Given :- chemical formula = Mn(NO3)2 To determine :- oxidation state of each element in the given…
Q: Calculate the oxidation state of each element in:
A: FOR ASSIGN OXIDATION STATE :IN NEUTRAL COMPOUNDS ALL OXDATION NUMBERS MUST ADD UP TO ZERO HYDROGEN…
Q: How do you determine the oxidation numbers for the following: a) N2H4 b) Cr2O7-2 c) NH4Cl…
A: You must know the oxidation state of the other elements other than main element for which we are…
Q: Predict the oxidation state of each atom in the following species: Ni(CO3)2 H3PO4 Na2O2
A: Predict the oxidation state of each atom in the following species--
Q: Some crystals of CuSO4 were dissolved in water. The color of the solution obtained would be:(a)…
A: An ionic bond can be defined as the electrostatic force of attraction between cation and anions.…
Q: What is the oxidation number of Cr in Na2Cr2O7 What is the oxidation number of K in KClO4 What is…
A:
Q: Assign an oxidation state to each atom NO2-
A:
Q: oxidation state of metal atom
A:
Q: What is the oxidation state of bromine in the compound HBrO3?
A: Oxidation state can be calculated as below : Sum of Oxidation state of each atom = charge of…
Q: Assign an oxidation state to each atom SO3
A: Assign an oxidation state to each atom in SO3.
Q: Good morning, Assign an oxidation state to each atom in each polyatomic ion, Cr2O7-2 Thank you,
A: Since we know that the oxygen has 6 electrons in its valence shell. Hence it will need 2 electron to…
Specify the oxidation state of the metal ions in the product
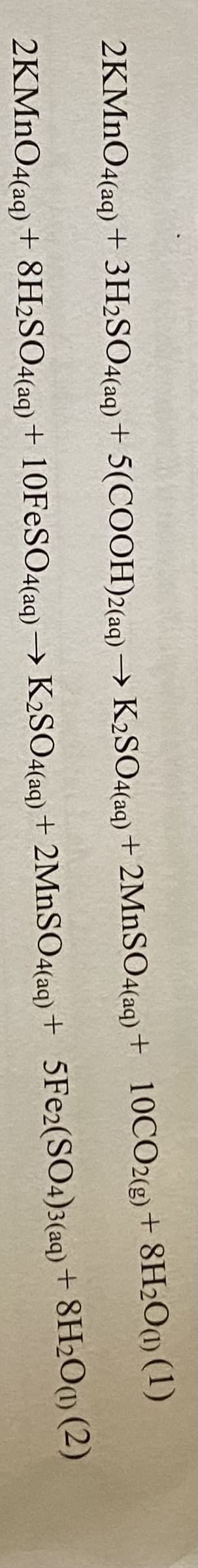
Step by step
Solved in 2 steps with 2 images

- 2 CoCl2(aq) + 8 NH3(aq) + H2O2(aq) + 2 NH4Cl(aq) 2 [Co(NH3)5Cl]Cl2(s) + 2 H2O(l) In a 50-mL Erlenmeyer flask, cobalt(II) chloride hexahydrate (1.075 g), an aqueous solution of ammonia (1.2 mL, 15 M), ammonium chloride (0.505 g), and hydrogen peroxide (1.0 mL, 30.0% (w/w), density = 1.10 g/mL) were combined. After completion of the reaction, [Co(NH3)5Cl]Cl2 (0.450 g) was isolated. What was the percent yield of the product?Beaker 0.00200 M Fe(NO3)3, mL 0.00200 M NaSCN, mL total volume, mL 1 3.000 2.000 10.00 2 3.000 3.000 10.00 3 3.000 4.000 10.00 4 3.000 5.000 10.00 5 (blank) 3.000 0.000 10.00 In Solutions 1-4 you are adding successively larger volumes of 0.00200M SCN- to the Fe3+ solution and diluting to 10.00 ml. Calculate the final diluted molarity of SCN- in solution #1 Your answer should have 3 sig figs =Calculate the ΔG°rxn using the following information.4 HNO3(g) + 5 N2H4(l) → 7 N2(g) + 12 H2O(l) ΔG°rxn = ?ΔH°f (kJ/mol)HNO3 = -133.9N2H4 = 50.6N2 = 0.0H2O = -285.8S°(J/mol∙K)HNO3 = 266.9N2H4 = 121.2N2 = 191.6H2O = 70.0
- 1. What is the mEq of silver nitrate? 170 0.0170 0.170 17.0 2. How much (mL) silver nitrate is required for the standardization? 4.995 49.950 49.590 45.990 * MW of silver nitrate = 170 g/mol * Please take note that Im looking for the silver nitrateWas the gradual color change observed when the sodium thiosulfate (Na2S2O3) crystal was added to the aqueous solution of KI/I2 in Station C evidence of a chemical or physical change? Group of answer choices chemical change physical changeSodium carbonate is a reagent that may be used to standardize acids. In such a standardization it was found that a 0.498-g sample of sodium carbonate required 22.6 mLmL mL of a sulfuric acid solution to reach the end point for the reaction. Na2CO3(aq)+H2SO4(aq)→H2O(l)+CO2(g)+Na2SO4(aq)Na2CO3(aq)+H2SO4(aq)→H2O(l)+CO2(g)+Na2SO4(aq) What is the molarity of the H2SO4H2SO4?
- Oceanic uptake of carbon dioxide is thus described:CO2 (g) + H2O ⇔ H2CO3, K = [H2CO3]/PCO2 = 3 x 10-2 M atm-1 H2CO3 ⇔ HCO3- + H+, K = [HCO3-][H+]/[H2CO3] = 9 x 10-7 moles/LHCO3- ⇔ CO32 - + H+, K = [CO32 -][H+]/[HCO3-] = 7 x 10-10 moles/LCharge balance equation:[H+] = [OH-] + [HCO3-] + 2[CO32 ] If the CO2 concentration in the atmosphere is 300 ppm, what is the pH of the ocean?1. 38Cu+Fe2+2Fe2+Option 42. 430.432Fe2+2Fe3+-0.343. 422Fe3+0.43-0.342Fe2+4. 452Fe3+2Fe2+0.43-0.345. 41Cu2-2Fe2+Fe2+Cu2+In a survey of 1000 large corporations, 250 said that, given a choice between a job candidate who smokes and an equally qualified nonsmoker, the nonsmoker would get the job (USA Today).(a) Let p represent the proportion of all corporations preferring a nonsmoking candidate. Find a point estimate for p.(b) Find a 0.95 confidence interval for p.(c) As a news writer, how would you report the survey results regarding the proportion of corporations that hire the equally qualified nonsmoker? What is the margin of error based on a 95% confidence interval?
- Mr. Roy, the principal of a reputed school organized a seminar in which he invited parents and principals to discuss the serious issue of diabetes and depression in students. They all resolved this issue by strictly banning the junk food in schools and to introduce healthy snacks and drinks’like soup, lassi, milk, etc. in school canteens. They also decided to make compulsory half an hour physical activities for the students in the morning assembly daily. After six months, Mr. Roy conducted the health survey in most of the schools and discovered a tremendous improvement in the health of students. After reading the above passage, answer the following: What are the values (at least two) displayed by Mr. Roy? As a student, how can you spread awareness about this issue? What are tranquilizers? Give an example. Why is the use of aspartame limited to cold foods and drinks?The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: 4 NH3 (g) + 7 O2 (g) → 4 NO2 (g) + 6 H2O (g) The combustion of 294 g of ammonia consumes __________ g of oxygen. Atomic weights: N=14.01, H=1.01, O=15.99. Input values only with 2 decimal places.The percentage of an additive in gasoline was measured six times with the following results: 0.13; 0.12; 0.16; 0.17; 0.20 and 0.11%. What is the 99% confidence interval for the additive percentage?

