Spotify Web Playe. O CHEMICAL REACTIONS 0/3 Solving moles-to-moles limiting reactant problems Solid aluminum (Al)and chlorine (Cl,) gas react to form solid aluminum chloride (AICI,). Suppose you have 11.0 mol of Al and 2.0 mol of Cl, in a reactor. Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol. Imol II
Spotify Web Playe. O CHEMICAL REACTIONS 0/3 Solving moles-to-moles limiting reactant problems Solid aluminum (Al)and chlorine (Cl,) gas react to form solid aluminum chloride (AICI,). Suppose you have 11.0 mol of Al and 2.0 mol of Cl, in a reactor. Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol. Imol II
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 44AP: A possible practical way to eliminate oxides of nitrogen(such as NO2 ) from automobile exhaust gases...
Related questions
Question
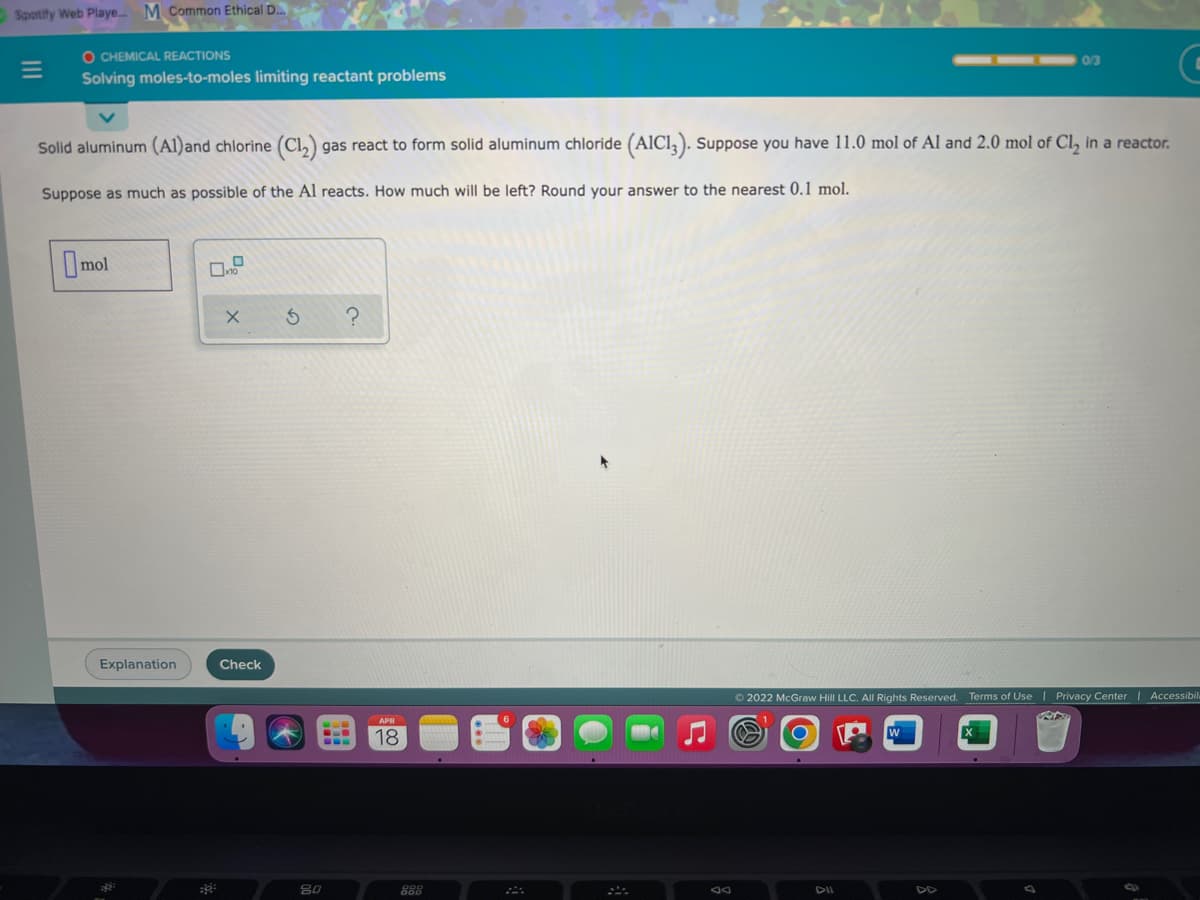
Transcribed Image Text:Spotify Web Playe.. M Common Ethical D.
O CHEMICAL REACTIONS
0/3
Solving moles-to-moles limiting reactant problems
Solid aluminum (Al)and chlorine (Cl,) gas react to form solid aluminum chloride (AICI, ). Suppose you have 11.0 mol of Al and 2.0 mol of Cl, in a reactor.
Suppose as much as possible of the Al reacts. How much will be left? Round your answer to the nearest 0.1 mol.
Imol
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibil
APR
603
18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co