State 1 1234 Pressure P (MPa) 0.14 0.80 0.80 0.14 Temperature T("C) -10 Specific volume v (m3/kg) Specific enthalpy h (kJ/kg) 278.0 95.48 State (saturated vapor, saturated liquid, etc.)? Saturated liquid
State 1 1234 Pressure P (MPa) 0.14 0.80 0.80 0.14 Temperature T("C) -10 Specific volume v (m3/kg) Specific enthalpy h (kJ/kg) 278.0 95.48 State (saturated vapor, saturated liquid, etc.)? Saturated liquid
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter1: Basic Modes Of Heat Transfer
Section: Chapter Questions
Problem 1.75P: Referring to Problem 1.74, how many kilograms of ice can a 3-ton refrigeration unit produce in a...
Related questions
Question
Yes in their book its in the appendix 1. But you can find the table for R134A anywhere in google.
Requirements: REQUEST TO YOU SOLVE ALL PARTS HANDWRITING THANKS FULL WARRANTY SOLUTIONS NEED . NO NEED GENERALIZED ANSWER OK
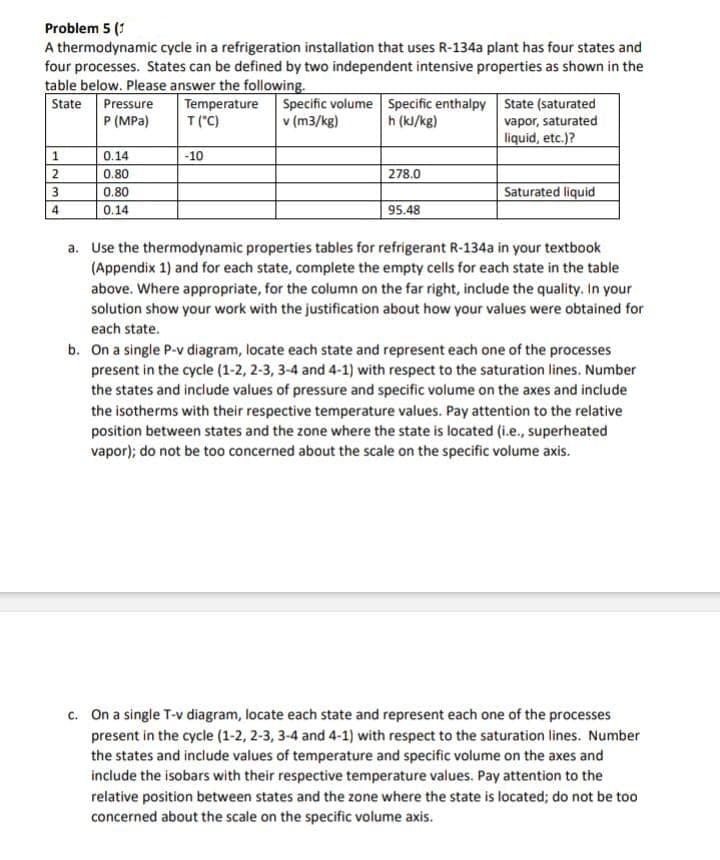
Transcribed Image Text:Problem 5 (1
A thermodynamic cycle in a refrigeration installation that uses R-134a plant has four states and
four processes. States can be defined by two independent intensive properties as shown in the
table below. Please answer the following.
Temperature
T("C)
State Pressure
P (MPa)
1
2
3
4
0.14
0.80
0.80
0.14
-10
Specific volume
v (m3/kg)
Specific enthalpy
h (kJ/kg)
278.0
95.48
State (saturated
vapor, saturated
liquid, etc.)?
Saturated liquid
a. Use the thermodynamic properties tables for refrigerant R-134a in your textbook
(Appendix 1) and for each state, complete the empty cells for each state in the table
above. Where appropriate, for the column on the far right, include the quality. In your
solution show your work with the justification about how your values were obtained for
each state.
b. On a single P-v diagram, locate each state and represent each one of the processes
present in the cycle (1-2, 2-3, 3-4 and 4-1) with respect to the saturation lines. Number
the states and include values of pressure and specific volume on the axes and include
the isotherms with their respective temperature values. Pay attention to the relative
position between states and the zone where the state is located (i.e., superheated
vapor); do not be too concerned about the scale on the specific volume axis.
c. On a single T-v diagram, locate each state and represent each one of the processes
present in the cycle (1-2, 2-3, 3-4 and 4-1) with respect to the saturation lines. Number
the states and include values of temperature and specific volume on the axes and
include the isobars with their respective temperature values. Pay attention to the
relative position between states and the zone where the state is located; do not be too
concerned about the scale on the specific volume axis.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 15 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning