stion Completion Status: Relative Abundance 100 90 80 70 60 50 40 30 20 10 0 21 Mass (amu) Consider the mass spectrum of neon above. It consists of an intense signal at mass number 20, an itty-bitty signal at 21, and a moderate signal at mass number 22. The heights of these signals are proportional to the number of counts at each mass number and, in tum, a reflection of the fractional abundance of the isotopes. The fractional abundance of each isotope is determined as follows: 20 22 1. The heights of all signals are measured with a metric ruler (in cm). 2. These heights are added together to give a total value. 3. The fractional abundance of an isotope is equal to the height of its signal divided by the sum of the heights of all the signals. Which of the following best represents the height measurements for each isqope? (scaling so the mass spectrum box is about 10 cm tall before measuring with a metric ruler) O 20 Ne 9.25 cm, 21 Ne 0.02 om, 22Ne 1.00 cm O 20 Ne 9 cm, 21 Ne 0.02 cm, 22Ne 1 cm O20Ne 3.5 cm, 21 Ne 0.01 cm, 22Ne 0.4 cm 20 Ne 91 cm, 21Ne 0.1 cm, 22Ne 10 cm
stion Completion Status: Relative Abundance 100 90 80 70 60 50 40 30 20 10 0 21 Mass (amu) Consider the mass spectrum of neon above. It consists of an intense signal at mass number 20, an itty-bitty signal at 21, and a moderate signal at mass number 22. The heights of these signals are proportional to the number of counts at each mass number and, in tum, a reflection of the fractional abundance of the isotopes. The fractional abundance of each isotope is determined as follows: 20 22 1. The heights of all signals are measured with a metric ruler (in cm). 2. These heights are added together to give a total value. 3. The fractional abundance of an isotope is equal to the height of its signal divided by the sum of the heights of all the signals. Which of the following best represents the height measurements for each isqope? (scaling so the mass spectrum box is about 10 cm tall before measuring with a metric ruler) O 20 Ne 9.25 cm, 21 Ne 0.02 om, 22Ne 1.00 cm O 20 Ne 9 cm, 21 Ne 0.02 cm, 22Ne 1 cm O20Ne 3.5 cm, 21 Ne 0.01 cm, 22Ne 0.4 cm 20 Ne 91 cm, 21Ne 0.1 cm, 22Ne 10 cm
Chapter29: Mass Spectrometry
Section: Chapter Questions
Problem 29.12QAP
Related questions
Question
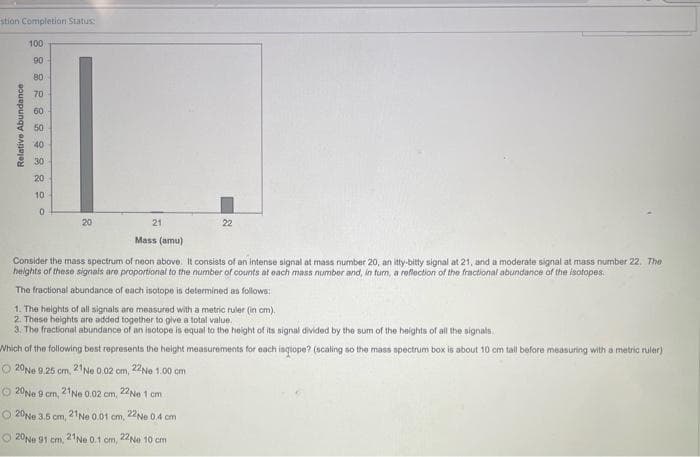
Transcribed Image Text:stion Completion Status:
Relative Abundance
100
90
80
70
60
50
40
30
20
10
0
20
21
22
Mass (amu)
Consider the mass spectrum of neon above. It consists of an intense signal at mass number 20, an itty-bitty signal at 21, and a moderate signal at mass number 22. The
heights of these signals are proportional to the number of counts at each mass number and, in turn, a reflection of the fractional abundance of the isotopes.
The fractional abundance of each isotope is determined as follows:
1. The heights of all signals are measured with a metric ruler (in cm).
2. These heights are added together to give a total value.
3. The fractional abundance of an isotope is equal to the height of its signal divided by the sum of the heights of all the signals
Which of the following best represents the height measurements for each isqope? (scaling so the mass spectrum box is about 10 cm tall before measuring with a metric ruler)
20Ne 9.25 cm, 21 Ne 0.02 cm, 22Ne 1.00 cm
O20Ne 9 cm, 21 Ne 0.02 cm, 22Ne 1 cm
O20Ne 3.5 cm,
21 Ne 0.01 cm, 22Ne 0.4 cm
20 Ne 91 cm, 21 Ne 0.1 cm, 22Ne 10 cm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning