Stoichiometry Gone Wrong - L Name Someone has been working on stoichiometry problems, but they've made some mistakes along the way. circle/highlight the mistakes and then work out the problem correctly (calculate the correct answer) and put in the shaded box with units!!! 2 Al + 3 Br, 1. How many moles of aluminum bromide would be produced from reacting 20 moles of aluminum? 2 AIBR, 20 moles Al 2 moles AlBr, 1 mole Al
Stoichiometry Gone Wrong - L Name Someone has been working on stoichiometry problems, but they've made some mistakes along the way. circle/highlight the mistakes and then work out the problem correctly (calculate the correct answer) and put in the shaded box with units!!! 2 Al + 3 Br, 1. How many moles of aluminum bromide would be produced from reacting 20 moles of aluminum? 2 AIBR, 20 moles Al 2 moles AlBr, 1 mole Al
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
100%
Transcribed Image Text:Stoich Gone Wrong (L) -
locument/d/1PrqdNDx-hBYyzKo-xfiM9-Jlq7EAaWe3GBt 13U3JD0/edit
Fraction Calculator
A Land of Permane...
bility
Last edit was 3 days ago
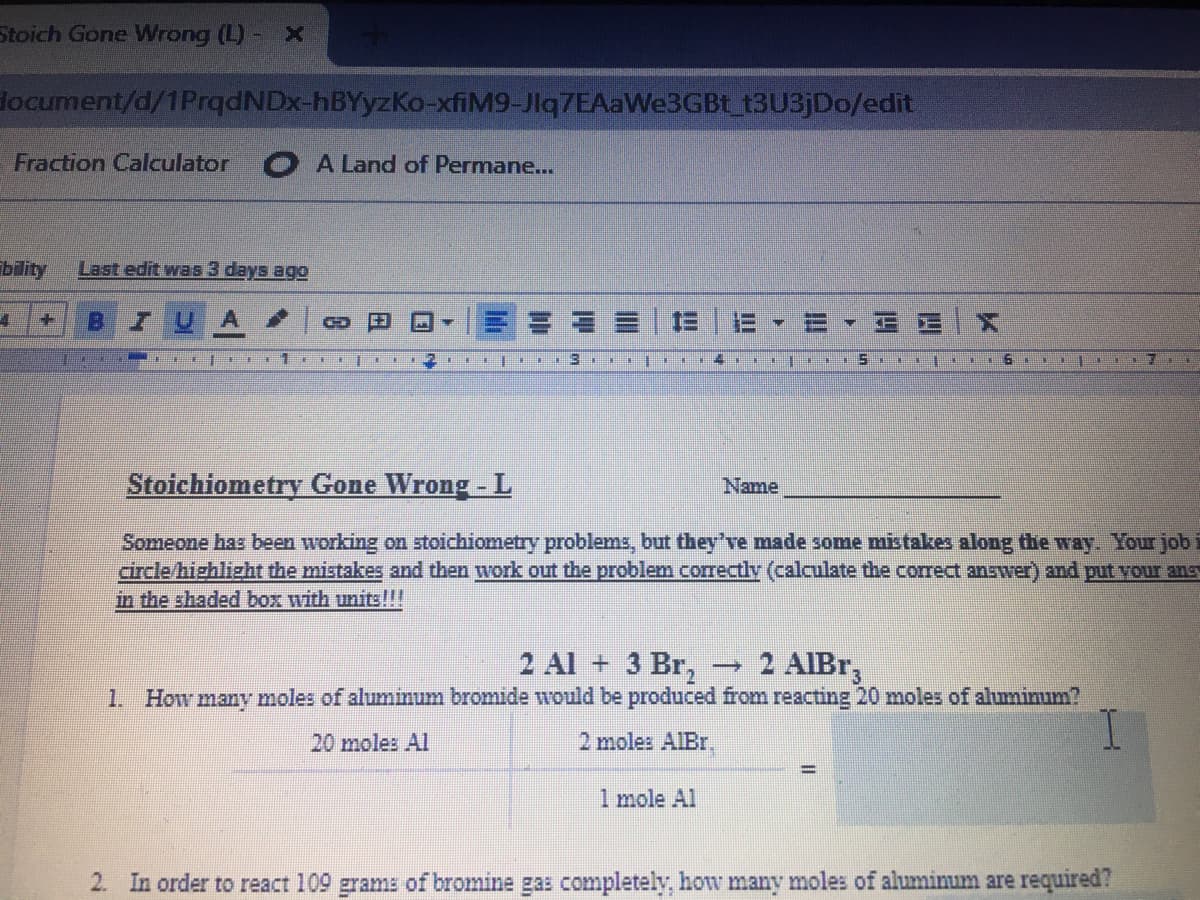
Stoichiometry Gone Wrong - L
Name
Someone has been working on stoichiometry problems, but they've made some mistakes along the way. Your job
circle/highlizht the mistakes and then work out the problem correctly (calculate the correct answer) and put vour ans
in the shaded box with units!!!
2 Al + 3 Br,
1. How many moles of aluminum bromide would be produced from reacting 20 moles of aluminum?
2 AIBT
一
20 moles Al
2 moles AIBr.
1 mole Al
2. In order to react 109 grams of bromine gas completely, how many moles of aluminum are required?

Transcribed Image Text:2. In order to react 109 grams of bromine gas completely, how many moles of aluminum are required?
3 moles Br.
2 moles Al
109 g Br.
159.8 g Br.
3 moles Br.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
