Stoichiometry Unit Test N2 (g) + 3H2 (g) 2NH3 (g) (S) Indicate how to calculate the mass of ammonia (NH3) that would be produced from the reaction of 2.56 x 1023 molecules of nitrogen gas with excess hydrogen gas? 2.56 1023 molec N2 The Periodic Table of the Elements
Stoichiometry Unit Test N2 (g) + 3H2 (g) 2NH3 (g) (S) Indicate how to calculate the mass of ammonia (NH3) that would be produced from the reaction of 2.56 x 1023 molecules of nitrogen gas with excess hydrogen gas? 2.56 1023 molec N2 The Periodic Table of the Elements
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.123PAE: 3.123 Most periodic tables provide molar masses with four or five significant figures for the...
Related questions
Question
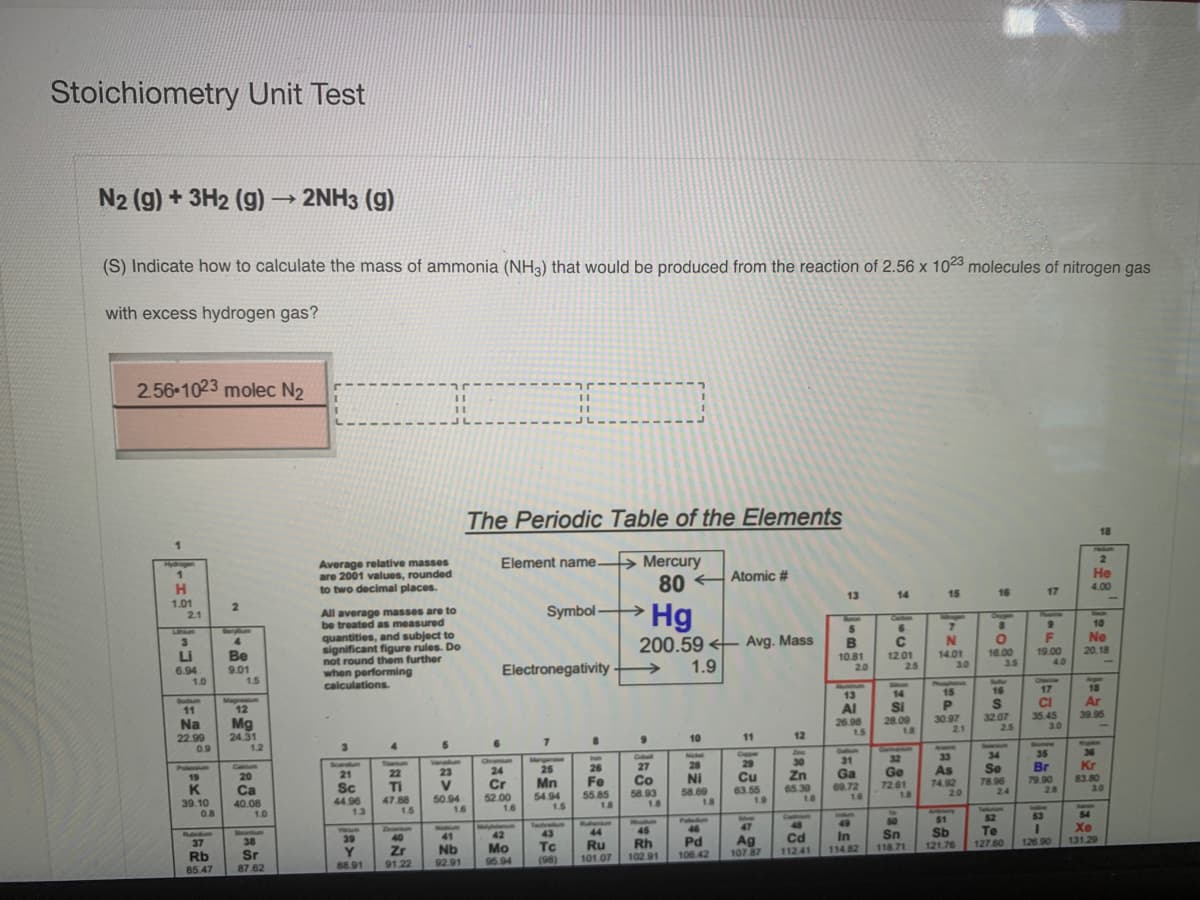
Transcribed Image Text:Stoichiometry Unit Test
N2 (g) + 3H2 (g)
2NH3 (g)
(S) Indicate how to calculate the mass of ammonia (NH3) that would be produced from the reaction of 2.56 x 1023 molecules of nitrogen gas
with excess hydrogen gas?
2.56 1023 molec N2
The Periodic Table of the Elements
18
TRIADS
> Mercury
80
Element name.
Average relative masses
are 2001 values, rounded
to two decimal places.
Hyogen
Atomic #
He
4.00
13
14
15
16
17
1.01
21
→Hg
2.
All average masses are to
be treated as measured
quantities, and subject to
significant figure rules. Do
not round them further
when performing
calculations.
Symbol -
Soap
10
Cabon
Lin
Ne
4.
Be
200.59 < Avg. Mass
19.00
4.0
20.18
LI
10.81
20
12.01
25
14.01
3.0
16.00
3.5
6.94
1.0
9.01
1.5
Electronegativity
1.9
Agan
18
ONemton
Phosan
15
16
17
14
SI
28.09
18
13
Sodum
11
Magneum
12
Al
26.98
CI
Ar
39.95
30.97
21
32.07
2.5
35.45
3.0
Na
22.99
0.9
Mg
24.31
12
10
11
12
15
3.
Dosee
35
Acwrc
Seeh
Oiel
Nel
Copper
33
34
36
Cu
32
30
Zn
65.39
1.6
31
Ga
09.72
16
Tourum
Vanadum
Chramu
28
29
Kr
Scandum
27
Pulaum
19
Br
79.90
28
Catium
Se
78.96
25
26
As
74.92
20
20
21
22
23
24
Ge
Cr
52.00
1.6
Mn
54.94
1.5
Fe
55.85
18
Co
58.93
18
NI
58.69
1.8
Cu
63.55
1.9
83.80
3.0
TI
Ca
40.08
.8
Sc
44.96
13
V
50.94
1.6
72.61
18
24
39.10
0.8
47.88
1.5
Autonary
51
Sb
121.76
Takm
1.0
53
54
Co
48
50
Sn
118.71
52
Noadas
45
Paled
46
Pd
49
Magholarm
42
Testram
43
Tc
47
Xe
131.29
44
Te
Rubidum
37
In
114.82
39
40
41
Cd
38
Sr
Ag
107 87
Mo
Ru
Rh
127.60
126 90
Y.
88.91
Zr
91.22
Nb
92.91
112.41
Rb
95.94
(98)
101.07
102.91
106 42
85.47
87.62
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning