Under certain circumstances, carbon dioxide, CO2 (g), can be made to react with hydrogen gas, H2 (g), to produce methane, CH (g), and water vapor, H20(g): Part A CO2(g) + 4H2 (g)→CH4(g) + 2H2O(g) How many moles of methane are produced when 36.6 moles of carbon dioxide gas react with excess hydrogen gas? Express your answer with the appropriate units. • View Available Hint(s) HÀ Value Units
Under certain circumstances, carbon dioxide, CO2 (g), can be made to react with hydrogen gas, H2 (g), to produce methane, CH (g), and water vapor, H20(g): Part A CO2(g) + 4H2 (g)→CH4(g) + 2H2O(g) How many moles of methane are produced when 36.6 moles of carbon dioxide gas react with excess hydrogen gas? Express your answer with the appropriate units. • View Available Hint(s) HÀ Value Units
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 55A
Related questions
Question
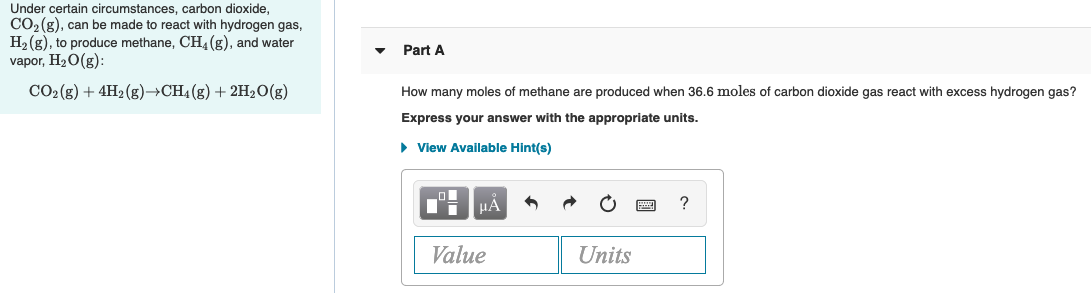
Transcribed Image Text:Under certain circumstances, carbon dioxide,
CO2 (g), can be made to react with hydrogen gas,
H2 (g), to produce methane, CH (g), and water
vapor, H20(g):
Part A
CO2(g) + 4H2 (g)→CH4(g) + 2H2O(g)
How many moles of methane are produced when 36.6 moles of carbon dioxide gas react with excess hydrogen gas?
Express your answer with the appropriate units.
• View Available Hint(s)
HÀ
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning