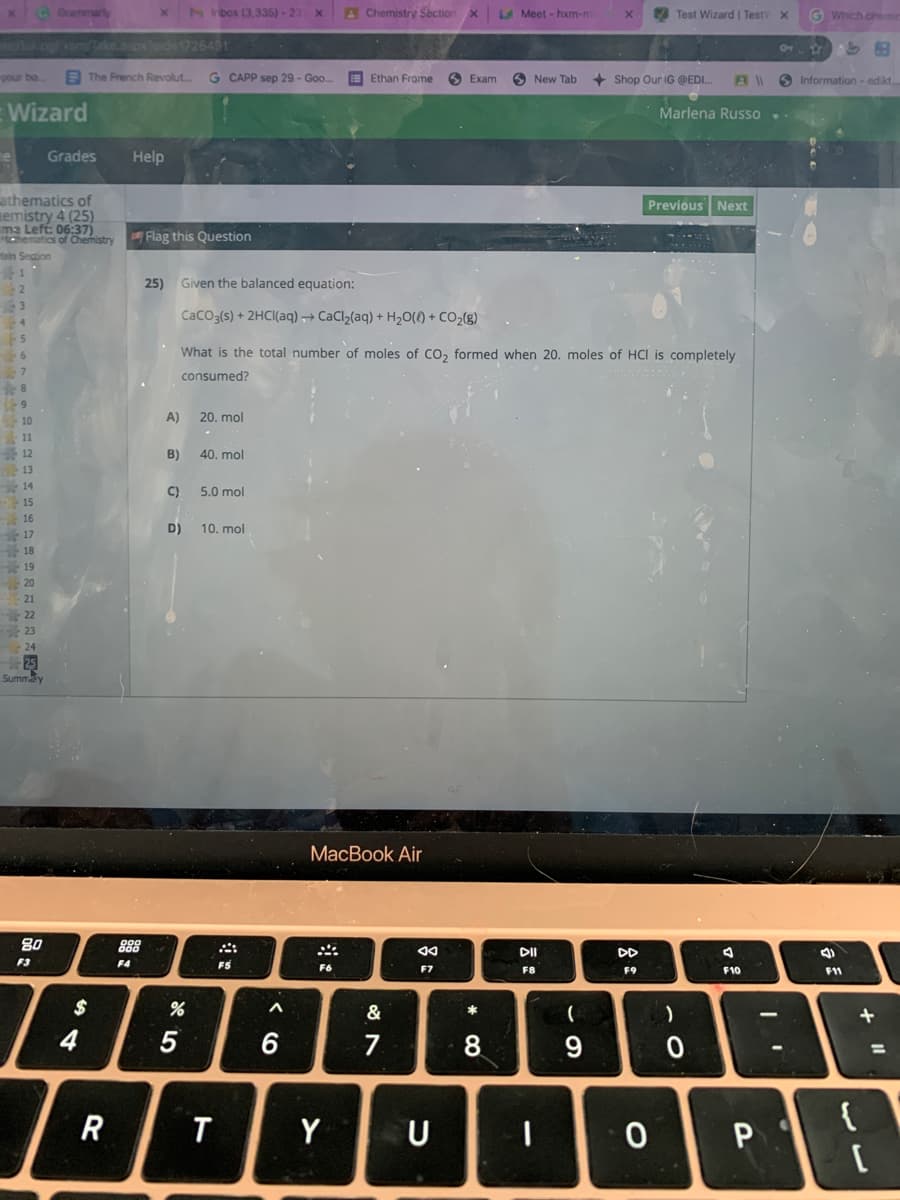
stry 25) Given the balanced equation: Caco;(s) + 2HCI(aq) → CaCl,(aq) + H2O() + CO2(8) What is the total number of moles of CO, formed when 20. moles of HCI is completely consumed? A) 20. mol B) 40. mol C) 5.0 mol
stry 25) Given the balanced equation: Caco;(s) + 2HCI(aq) → CaCl,(aq) + H2O() + CO2(8) What is the total number of moles of CO, formed when 20. moles of HCI is completely consumed? A) 20. mol B) 40. mol C) 5.0 mol
Chapter33: High-performance Liquid Chromatography
Section: Chapter Questions
Problem 33.17QAP
Related questions
Question
Transcribed Image Text:G Grammary
M rbox (3,335) - 23
A Chemistry Section x
LA Meet - hxm-m
Test Wizard | Testy x
G Which onemi
kepde1726491
your bo
The French Revolut
G CAPP sep 29 - Go..
E Ethan Frome
O Exam
6 New Tab
+ Shop Our IG @EDI..
O Information - edikt..
Wizard
Marlena Russo .
Grades
Help
athematics of
emistry 4 (25)
ma Left: 06:37)
Previous Next
Flag this Question
chematics of Chemistry
ain Section
25)
Given the balanced equation:
CaCO3(s) + 2HCI(aq) → CaClz(aq) + H20(1) + CO2(g)
What is the total number of moles of Co, formed when 20. moles of HCI is completely
consumed?
A)
20. mol
12
B)
40. mol
13
14
C)
5.0 mol
15
16
D)
10. mol
17
18
Summay
MacBook Air
80
888
DII
DD
F3
F4
FS
F6
F7
F8
F9
F10
11
$
&
7
8.
9.
R
T
Y
U
{
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
