Summarize each of the gas laws (Boyle's law, Charles's law, and Avogadro's law). Drag the terms on the left to the appropriate blanks on the right to complete the sentences. Reset Help Boyle's law states that the volume of the gas is proportional to the pressure of the Charles's law gas, when the temperature and number of moles are kept constant. Avogadro's law states that the volume of a gas is proportional to the number of moles of the gas, when the pressure and temperature are kept constant. It also states that at constant pressure and temperature, an equal number of moles of different gases will occupy inversely directly volume. Kelvin Celsius states that the volume of a gas is proportional to the temperature of the gas, when the pressure and number of moles are kept constant. All temperatures must be in units the same of when used in gas law calculations. different
Summarize each of the gas laws (Boyle's law, Charles's law, and Avogadro's law). Drag the terms on the left to the appropriate blanks on the right to complete the sentences. Reset Help Boyle's law states that the volume of the gas is proportional to the pressure of the Charles's law gas, when the temperature and number of moles are kept constant. Avogadro's law states that the volume of a gas is proportional to the number of moles of the gas, when the pressure and temperature are kept constant. It also states that at constant pressure and temperature, an equal number of moles of different gases will occupy inversely directly volume. Kelvin Celsius states that the volume of a gas is proportional to the temperature of the gas, when the pressure and number of moles are kept constant. All temperatures must be in units the same of when used in gas law calculations. different
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 76A
Related questions
Question
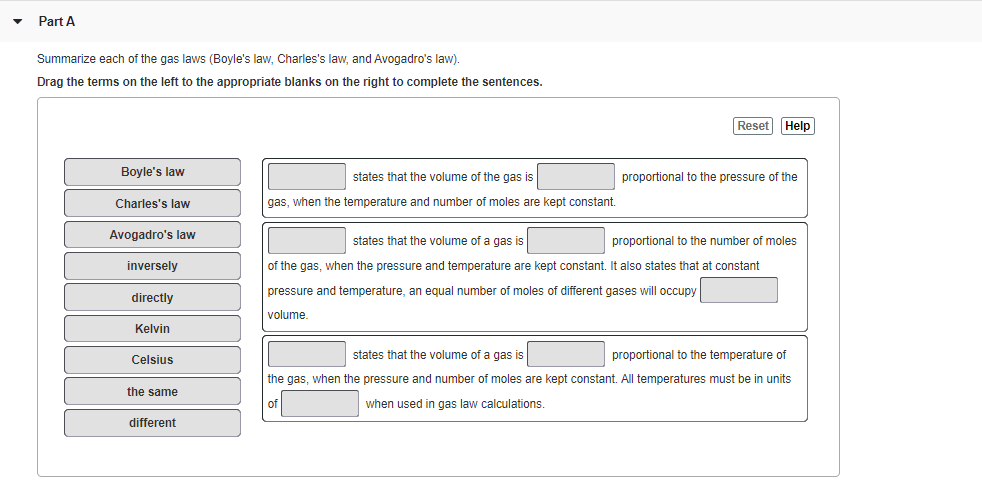
Transcribed Image Text:Part A
Summarize each of the gas laws (Boyle's law, Charles's law, and Avogadro's law).
Drag the terms on the left to the appropriate blanks on the right to complete the sentences.
Reset
Help
Boyle's law
states that the volume of the gas is
proportional to the pressure of the
Charles's law
gas, when the temperature and number of moles are kept constant.
Avogadro's law
states that the volume of a gas is
proportional to the number of moles
inversely
of the gas, when the pressure and temperature are kept constant. It also states that at constant
pressure and temperature, an equal number of moles of different gases will occupy
directly
volume.
Kelvin
states that the volume of a gas is
proportional to the temperature of
Celsius
the gas, when the pressure and number of moles are kept constant. All temperatures must be in units
the same
when used in gas law calculations.
different
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning