suo-ppy Help Last edit was seconds ago BIUA Arial 11 +. Roland Marris Ionization describes the process in which an electron is removed from an atom in the gaseous state. The amount of energy required to remove the outer electron is called the first ionization energy. Successive electrons can be removed as well. The following cquations show the first and second NigN'tg)+e NiglN ) +e ionization for the nitrogen atom. First lonization Second lonization Bohr's model of sodium. The Cler olocteon is aactod to te eucle.s y opposne charga. Itisse aheKd by the Dore olectrors First lonization Energy The ionization cnergy varies depenling on how strongly cach electron is held to the mucleus. This attractive force is related to thre Yactors. First, the distance bctween the clectron and the nucleus affects the electrostatic attraction: the closer the clectron the stronger the force. Second. the number of protons in the mucleus can affect the attraction of the electrons. The more electrons there are the greater the attraction. Finally. the electrons between the nucleus and the outermost electron are responsible for a repulsion called shielding. 1 Use the graph to list the ionization energies forthe noble gases Element He Ne Ar Kr Xe Slement h8A Energy 2 Make a statement of the trend observed in ionization energy as you go down the periodic table (from He-Ne-Ar -Kr-Xe)
suo-ppy Help Last edit was seconds ago BIUA Arial 11 +. Roland Marris Ionization describes the process in which an electron is removed from an atom in the gaseous state. The amount of energy required to remove the outer electron is called the first ionization energy. Successive electrons can be removed as well. The following cquations show the first and second NigN'tg)+e NiglN ) +e ionization for the nitrogen atom. First lonization Second lonization Bohr's model of sodium. The Cler olocteon is aactod to te eucle.s y opposne charga. Itisse aheKd by the Dore olectrors First lonization Energy The ionization cnergy varies depenling on how strongly cach electron is held to the mucleus. This attractive force is related to thre Yactors. First, the distance bctween the clectron and the nucleus affects the electrostatic attraction: the closer the clectron the stronger the force. Second. the number of protons in the mucleus can affect the attraction of the electrons. The more electrons there are the greater the attraction. Finally. the electrons between the nucleus and the outermost electron are responsible for a repulsion called shielding. 1 Use the graph to list the ionization energies forthe noble gases Element He Ne Ar Kr Xe Slement h8A Energy 2 Make a statement of the trend observed in ionization energy as you go down the periodic table (from He-Ne-Ar -Kr-Xe)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 70SCQ: The ionization of the hydrogen atom can be calculated from Bohr's equation for the electron energy....
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
100%
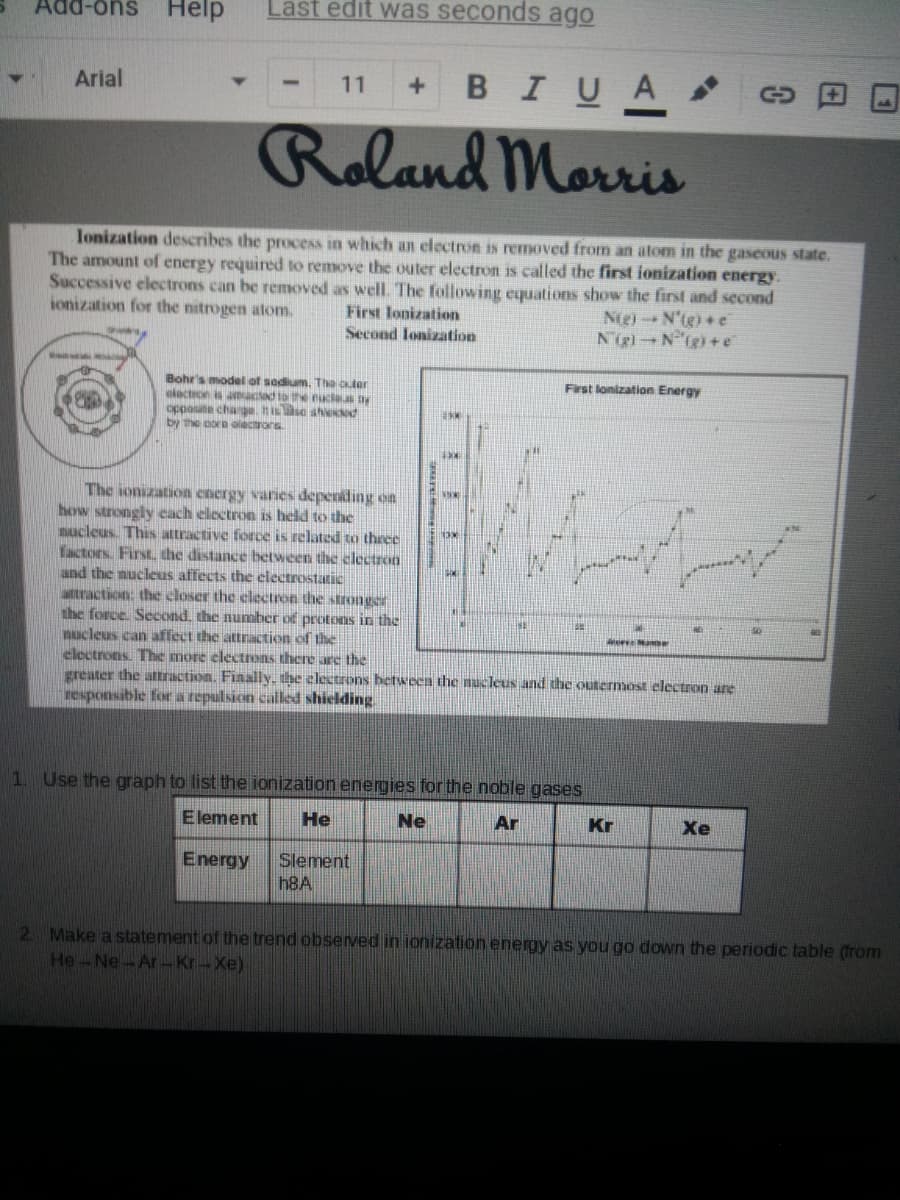
Transcribed Image Text:suo-ppy
Help
Last edit was seconds ago
BIUA
Arial
11
+.
Roland Morris
Ionization describes the process in which an electron is removed from an atom in the gaseous state.
The amount of energy required to remove the outer electron is called the first ionization energy.
Successive electrons can be removed as well. The following cquations show the first and second
Nig)N'tg) +e
Nigl N (g) +e
ionization for the nitrogen atom.
First lonization
Second lonization
Bohr's model of sodium. The Cler
oloctron s amactd tote eucle.s y
opposite charga. It issa aheKd
by the oore aliectrors
First lonization Energy
The ionization cnergy varies depending on
how strongly cach electron is held to the
mucleus. This attractive force is related to three
Yactors. First, the distance bctween the clectron
and the nucleus affects the electrostatic
attraction: the closer the clectron the stronger
the force. Second. the number of protons in the
mucleus can affect the attraction of the
electrons. The more electrons there are the
greater the attraction. Finally. the electrons hetween the nucleus and the outermost electron are
responsible for a repulsion called shielding
1 Use the graph to list the ionization energies forthe noble gases
Element
He
Ne
Ar
Kr
Xe
Slement
h8A
Energy
2. Make a statement of the trend observed in ionization energy as you go down the periodic table (from
He-Ne-Ar -Kr-Xe)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning