Suppose 2.00 mol of an ideal, monatomic gas is initially at a pressure of 3.00 atm and a temperature T = 350 K. It is expanded irreversibly and adiabatically (q = 0) against a constant external pressure of 1.00 atm until the volume has doubled. (a) Calculate the final volume. (b) Calculate w, q, and AU for this process, in joules. (c) Calculate the final temperature of the gas.
Suppose 2.00 mol of an ideal, monatomic gas is initially at a pressure of 3.00 atm and a temperature T = 350 K. It is expanded irreversibly and adiabatically (q = 0) against a constant external pressure of 1.00 atm until the volume has doubled. (a) Calculate the final volume. (b) Calculate w, q, and AU for this process, in joules. (c) Calculate the final temperature of the gas.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 19P
Related questions
Question
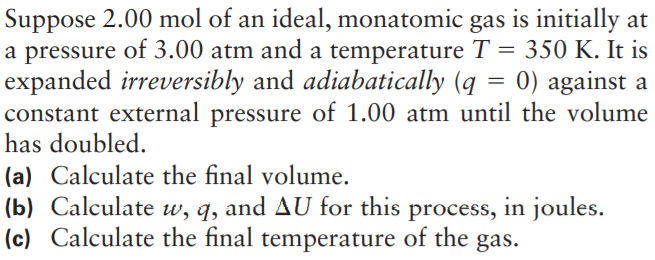
Transcribed Image Text:Suppose 2.00 mol of an ideal, monatomic gas is initially at
a pressure of 3.00 atm and a temperature T = 350 K. It is
expanded irreversibly and adiabatically (q = 0) against a
constant external pressure of 1.00 atm until the volume
has doubled.
(a) Calculate the final volume.
(b) Calculate w, q, and AU for this process, in joules.
(c) Calculate the final temperature of the
gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning