Suppose a 250. mL flask is filled with 0.90 mol of N, and 0.50 mol of NO. The following reaction becomes possible: N2(g) +O,(g) - 2NO(g) The equilibrium constant K for this reaction is 0.212 at the temperature of the flask. Calculate the equilibrium molarity of N2. Round your answer to two decimal places.
Suppose a 250. mL flask is filled with 0.90 mol of N, and 0.50 mol of NO. The following reaction becomes possible: N2(g) +O,(g) - 2NO(g) The equilibrium constant K for this reaction is 0.212 at the temperature of the flask. Calculate the equilibrium molarity of N2. Round your answer to two decimal places.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.76EP: Calculate the value of the equilibrium constant for the reaction N2(g)+2O2(g)2NO2(g) if the...
Related questions
Question
100%
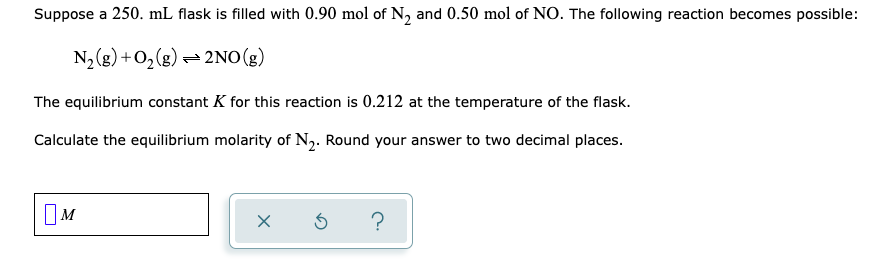
Transcribed Image Text:Suppose a 250. mL flask is filled with 0.90 mol of N, and 0.50 mol of NO. The following reaction becomes possible:
N2(g) +O,(g) - 2NO(g)
The equilibrium constant K for this reaction is 0.212 at the temperature of the flask.
Calculate the equilibrium molarity of N2. Round your answer to two decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning