Suppose a 250. mL flask is filled with 1.2 mol of CHCI,, 0.10 mol of HCl and 1.7 mol of CCl. The following reaction becomes possible: Cl, (g) + CHCI, (g) - HCI(g) +CCl,(g) The equilibrium constant K for this reaction is 5.74 at the temperature of the flask. Calculate the equilibrium molarity of Cl,. Round your answer to two decimal places.
Suppose a 250. mL flask is filled with 1.2 mol of CHCI,, 0.10 mol of HCl and 1.7 mol of CCl. The following reaction becomes possible: Cl, (g) + CHCI, (g) - HCI(g) +CCl,(g) The equilibrium constant K for this reaction is 5.74 at the temperature of the flask. Calculate the equilibrium molarity of Cl,. Round your answer to two decimal places.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 17A
Related questions
Question
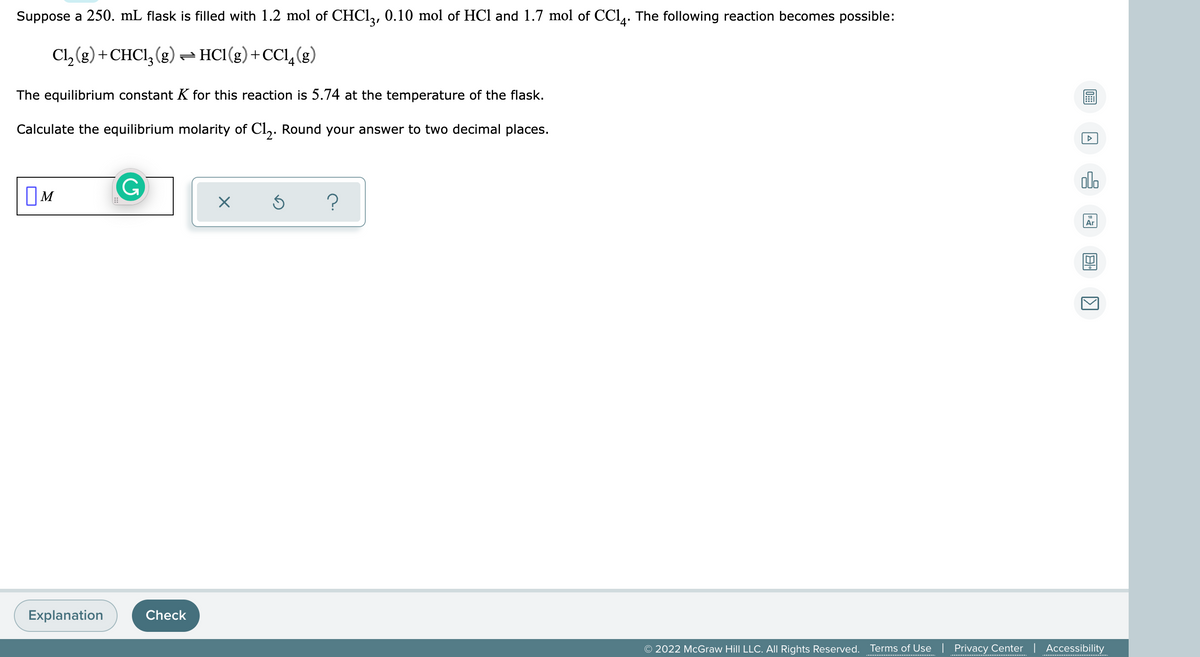
Transcribed Image Text:Suppose a 250. mL flask is filled with 1.2 mol of CHCI,, 0.10 mol of HCl and 1.7 mol of CCl,. The following reaction becomes possible:
Cl, (g) +CHCI, (g) - HC1(g)+CCl,(g)
The equilibrium constant K for this reaction is 5.74 at the temperature of the flask.
Calculate the equilibrium molarity of Cl,. Round your answer to two decimal places.
G
dlo
OM
Ar
Explanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use| Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning