Suppose a boil water notice is sent out advising all residents in the area to boil their water before drinking it or using it for cooking. You need to boil 12.0 L of water using your natural gas (primarily methane) stove. What volume of natural gas is needed to boil the water if only 19.1% of the heat generated goes towards heating the water. Assume the density of methane is 0.668 g/L, the density of water is 1.00 g/mL, and that the water has an initial temperature of 21.3 °C. Enthalpy of formation values can be found in this table. Assume that gaseous water is formed in the combustion of methane. 549.69 volume of methane: Incorrect
Suppose a boil water notice is sent out advising all residents in the area to boil their water before drinking it or using it for cooking. You need to boil 12.0 L of water using your natural gas (primarily methane) stove. What volume of natural gas is needed to boil the water if only 19.1% of the heat generated goes towards heating the water. Assume the density of methane is 0.668 g/L, the density of water is 1.00 g/mL, and that the water has an initial temperature of 21.3 °C. Enthalpy of formation values can be found in this table. Assume that gaseous water is formed in the combustion of methane. 549.69 volume of methane: Incorrect
Chapter10: Energy
Section: Chapter Questions
Problem 65A
Related questions
Question
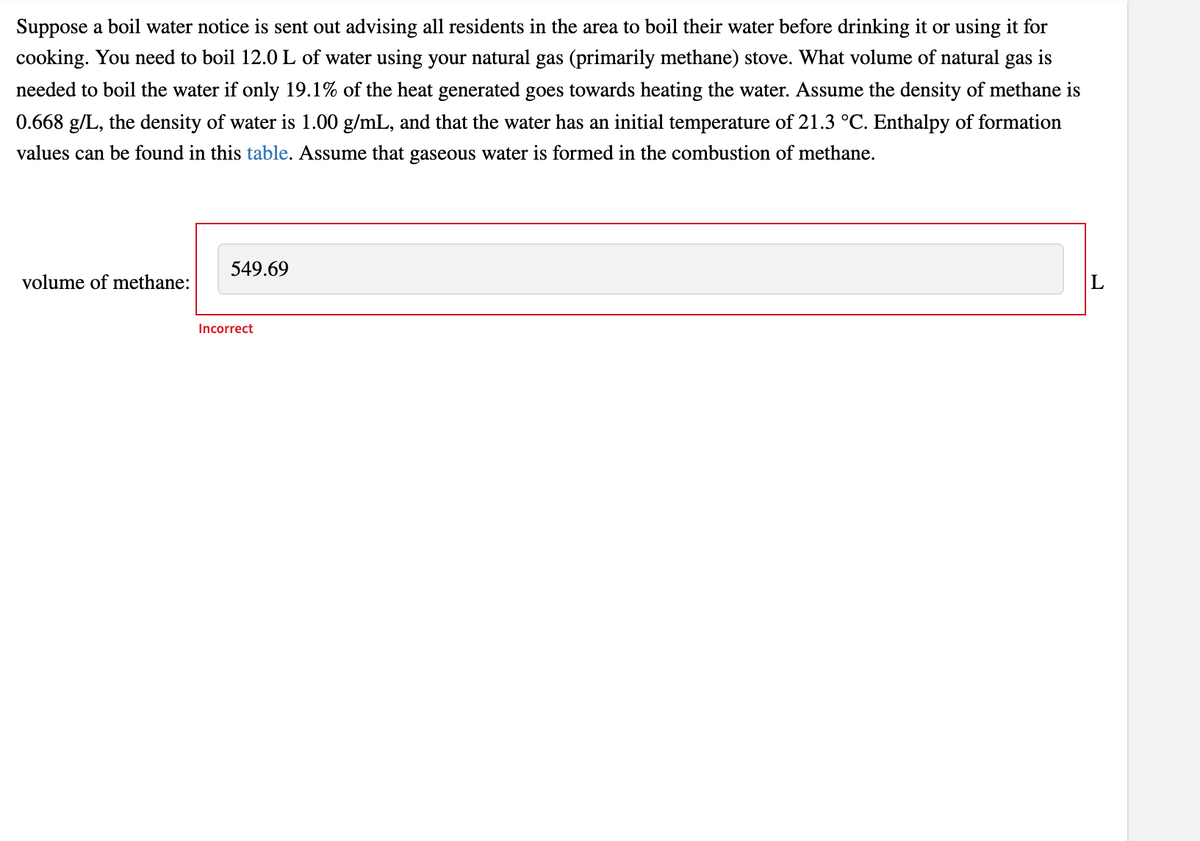
Transcribed Image Text:Suppose a boil water notice is sent out advising all residents in the area to boil their water before drinking it or using it for
cooking. You need to boil 12.0 L of water using your natural gas (primarily methane) stove. What volume of natural
gas
is
needed to boil the water if only 19.1% of the heat generated goes towards heating the water. Assume the density of methane is
0.668 g/L, the density of water is 1.00 g/mL, and that the water has an initial temperature of 21.3 °C. Enthalpy of formation
values can be found in this table. Assume that gaseous water is formed in the combustion of methane.
549.69
volume of methane:
L
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax