When water cools from 10 °C and then freezes to become ice, which of the following best describes describe the heat flow between the system and surroundings? A) The energy of the universe is decreasing because the water is cold. B) The energy of the universe is increasing because the energy had to be removed from the water C) The water is absorbing energy as its temperature decreases, and that energy was released by the surroundings. D) The water is releasing energy as its temperature decreases, and that energy is absorbed by the surroundings.
When water cools from 10 °C and then freezes to become ice, which of the following best describes describe the heat flow between the system and surroundings? A) The energy of the universe is decreasing because the water is cold. B) The energy of the universe is increasing because the energy had to be removed from the water C) The water is absorbing energy as its temperature decreases, and that energy was released by the surroundings. D) The water is releasing energy as its temperature decreases, and that energy is absorbed by the surroundings.
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 4STP
Related questions
Question
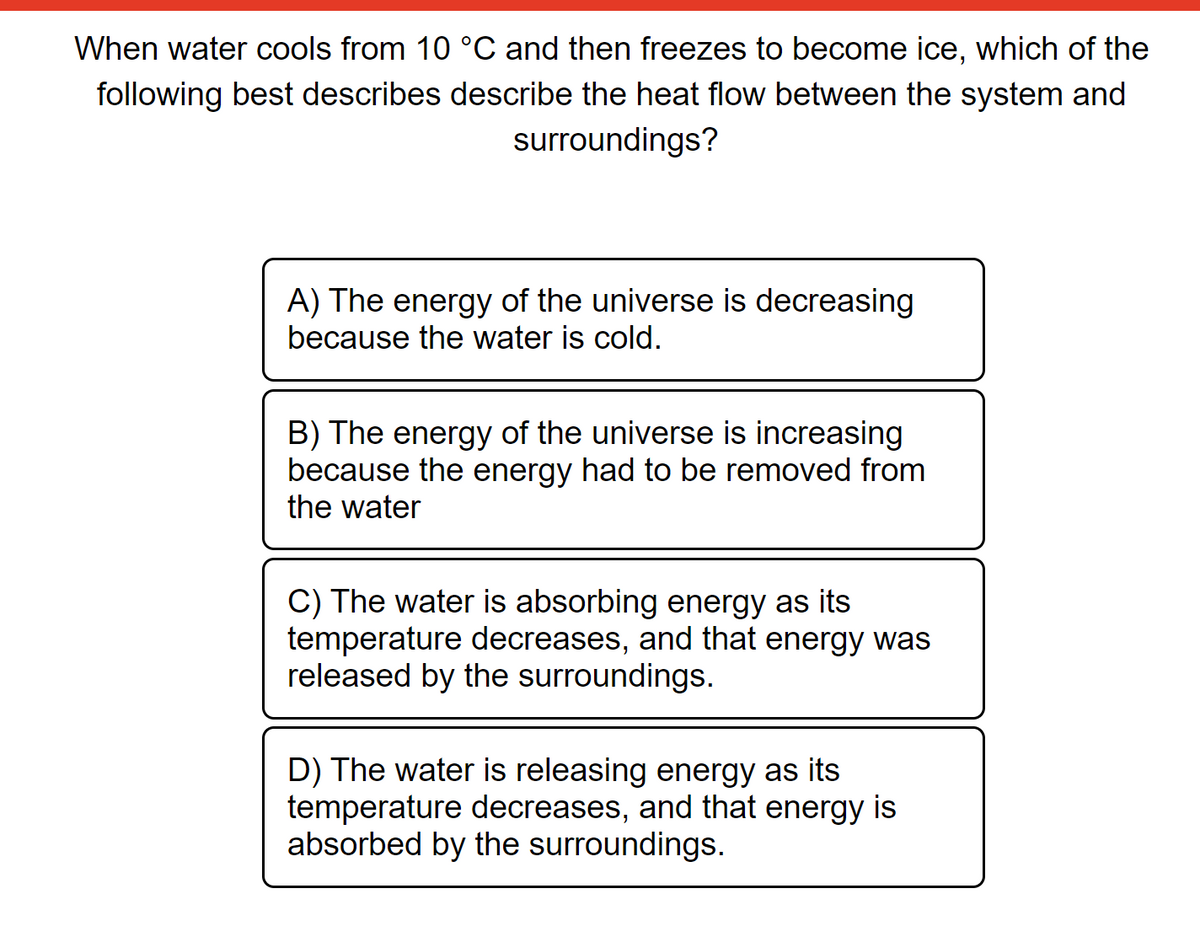
Transcribed Image Text:When water cools from 10 °C and then freezes to become ice, which of the
following best describes describe the heat flow between the system and
surroundings?
A) The energy of the universe is decreasing
because the water is cold.
B) The energy of the universe is increasing
because the energy had to be removed from
the water
C) The water is absorbing energy as its
temperature decreases, and that energy was
released by the surroundings.
D) The water is releasing energy as its
temperature decreases, and that energy is
absorbed by the surroundings.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning