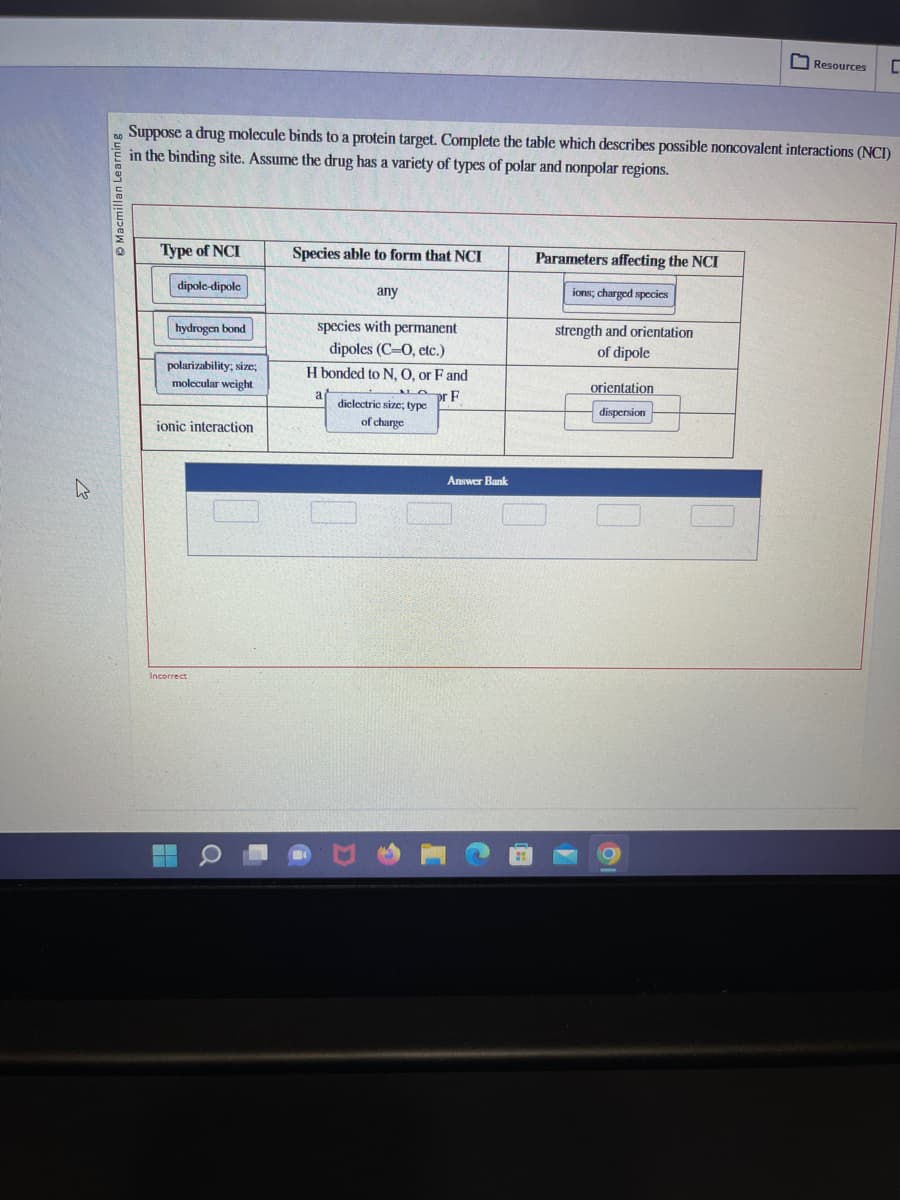
Suppose a drug molecule binds to a protein target. Complete the table which describes possible noncovalent interactions (N in the binding site. Assume the drug has a variety of types of polar and nonpolar regions. Type of NCI dipole-dipole hydrogen bond polarizability, size; molecular weight ionic interaction Species able to form that NCI any species with permanent dipoles (C-O, etc.) H bonded to N, O, or F and F a dielectric size; type of charge Parameters affecting the NCI ions; charged species strength and orientation of dipole orientation dispersion
Suppose a drug molecule binds to a protein target. Complete the table which describes possible noncovalent interactions (N in the binding site. Assume the drug has a variety of types of polar and nonpolar regions. Type of NCI dipole-dipole hydrogen bond polarizability, size; molecular weight ionic interaction Species able to form that NCI any species with permanent dipoles (C-O, etc.) H bonded to N, O, or F and F a dielectric size; type of charge Parameters affecting the NCI ions; charged species strength and orientation of dipole orientation dispersion
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 10CTQ
Related questions
Question
How can we match these?
Transcribed Image Text:Macmillan Learning
Suppose a drug molecule binds to a protein target. Complete the table which describes possible noncovalent interactions (NCI)
in the binding site. Assume the drug has a variety of types of polar and nonpolar regions.
Type of NCI
dipole-dipole
hydrogen bond
polarizability, size;
molecular weight
ionic interaction
Incorrect
Species able to form that NCI
any
species with permanent
dipoles (C-O, etc.)
H bonded to N, O, or F and
F
dielectric size; type
of charge
Answer Bank
Parameters affecting the NCI
ions; charged species
strength and orientation
of dipole
orientation
dispersion
Resources C
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
