Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies 25.8 L at 75.0°C and 41.1 kPa. A) 95.0°C B) 85.1°C C) 65.2°C D) 72.9°C E) 77.2°C A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr. m What is its volume at STP? 2521 B) 22.4 L C) 34.3 L D) 47.7 L E) 31.1 L
Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies 25.8 L at 75.0°C and 41.1 kPa. A) 95.0°C B) 85.1°C C) 65.2°C D) 72.9°C E) 77.2°C A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr. m What is its volume at STP? 2521 B) 22.4 L C) 34.3 L D) 47.7 L E) 31.1 L
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
100%
How would you answer these questions?
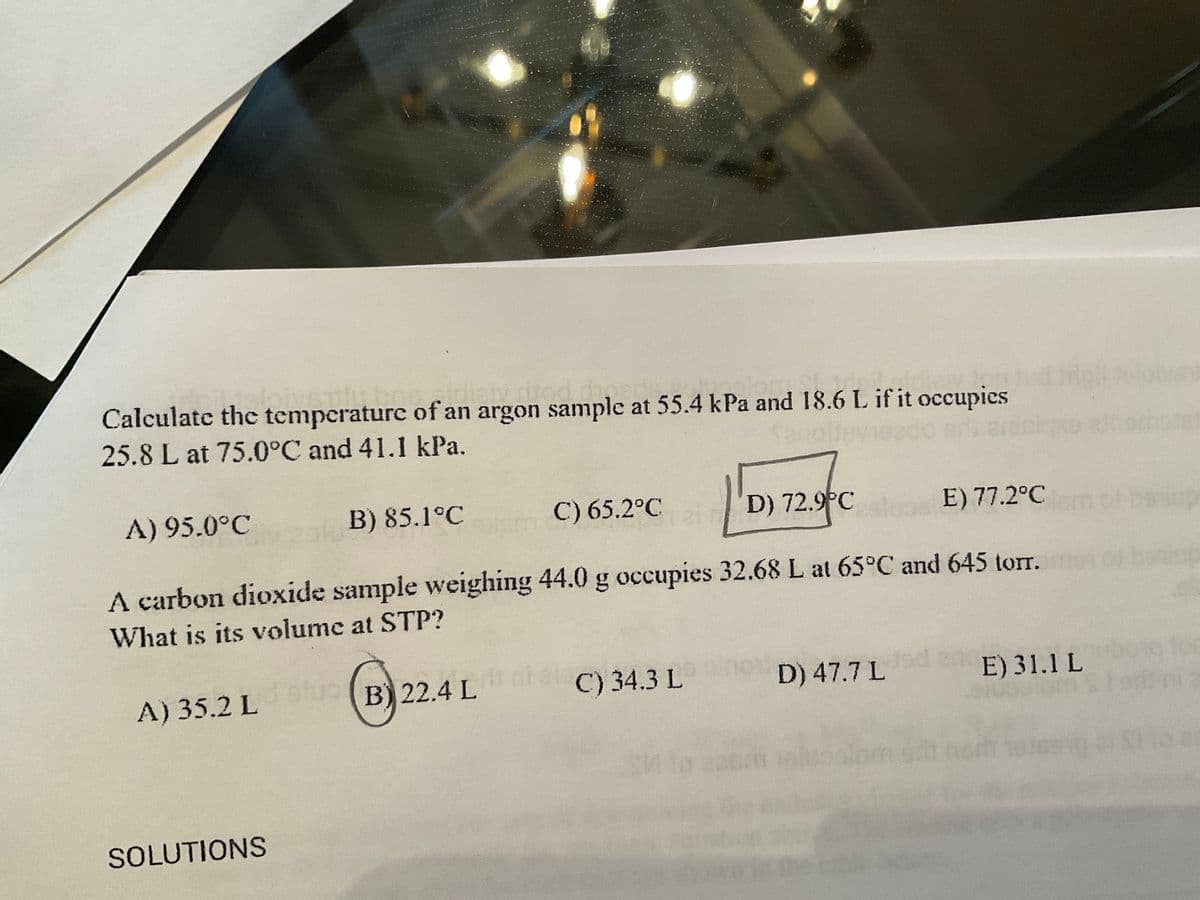
Transcribed Image Text:Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies
25.8 L at 75.0°C and 41.1 kPa.
A) 95.0°C
C) 65.2°C
SOLUTIONS
B) 85.1°C
D) 72.9°C
E) 77.2°Clem of
A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr.
What is its volume at STP?
A) 35.2 Lalu (B) 22.4 LC) 34.3 L
n
D) 47.7 L
do
ed
E) 31.1 L
518
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning