Suppose the decomposition of dinitrogen monoxide proceeds by the following mechanism: elementary reaction N₂O(g) N₂(g) + O(g) k₁ 2 N₂O(g) + O(g) N₂(g) + O₂(g) k₂ Suppose also k₁«k₂. That is, the first step is much slower than the second. step - Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of K₁, K₂, and (if necessary) the rate constants k.₁ and K-2 for the rouoren of the two 0 rate = k k = 0 rate constant
Suppose the decomposition of dinitrogen monoxide proceeds by the following mechanism: elementary reaction N₂O(g) N₂(g) + O(g) k₁ 2 N₂O(g) + O(g) N₂(g) + O₂(g) k₂ Suppose also k₁«k₂. That is, the first step is much slower than the second. step - Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of K₁, K₂, and (if necessary) the rate constants k.₁ and K-2 for the rouoren of the two 0 rate = k k = 0 rate constant
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.43E
Related questions
Question
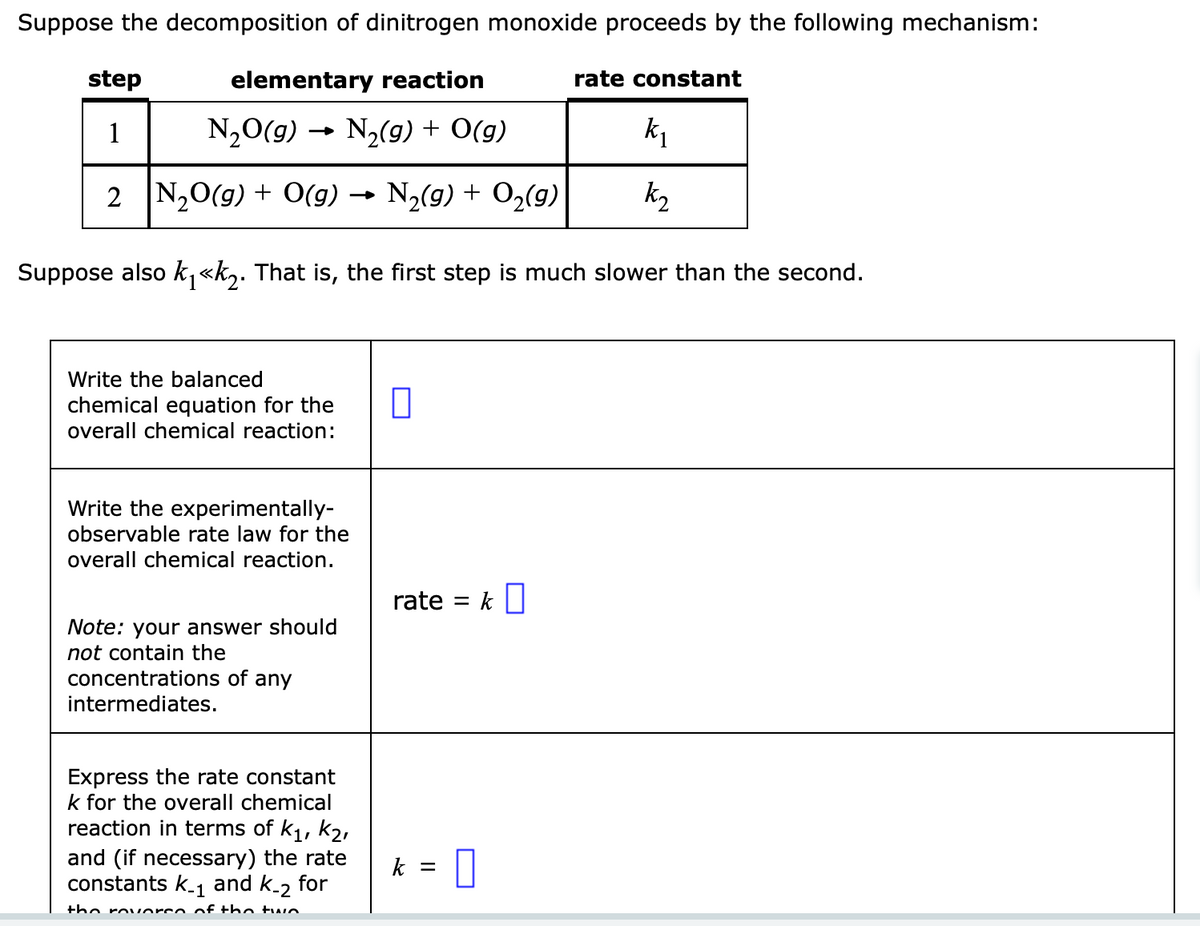
Transcribed Image Text:Suppose the decomposition of dinitrogen monoxide proceeds by the following mechanism:
elementary reaction
N₂O(g) N₂(g) + O(g)
k₁
2 N₂O(g) + O(g) → N₂(g) + O₂(g)
k₂
Suppose also k₁<<k₂. That is, the first step is much slower than the second.
step
1
Write the balanced
chemical equation for the
overall chemical reaction:
Write the experimentally-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of k₁, K₂,
and (if necessary) the rate
constants k_₁ and k-2 for
the rovorce of the two
0
rate = k
k
- 0
=
rate constant
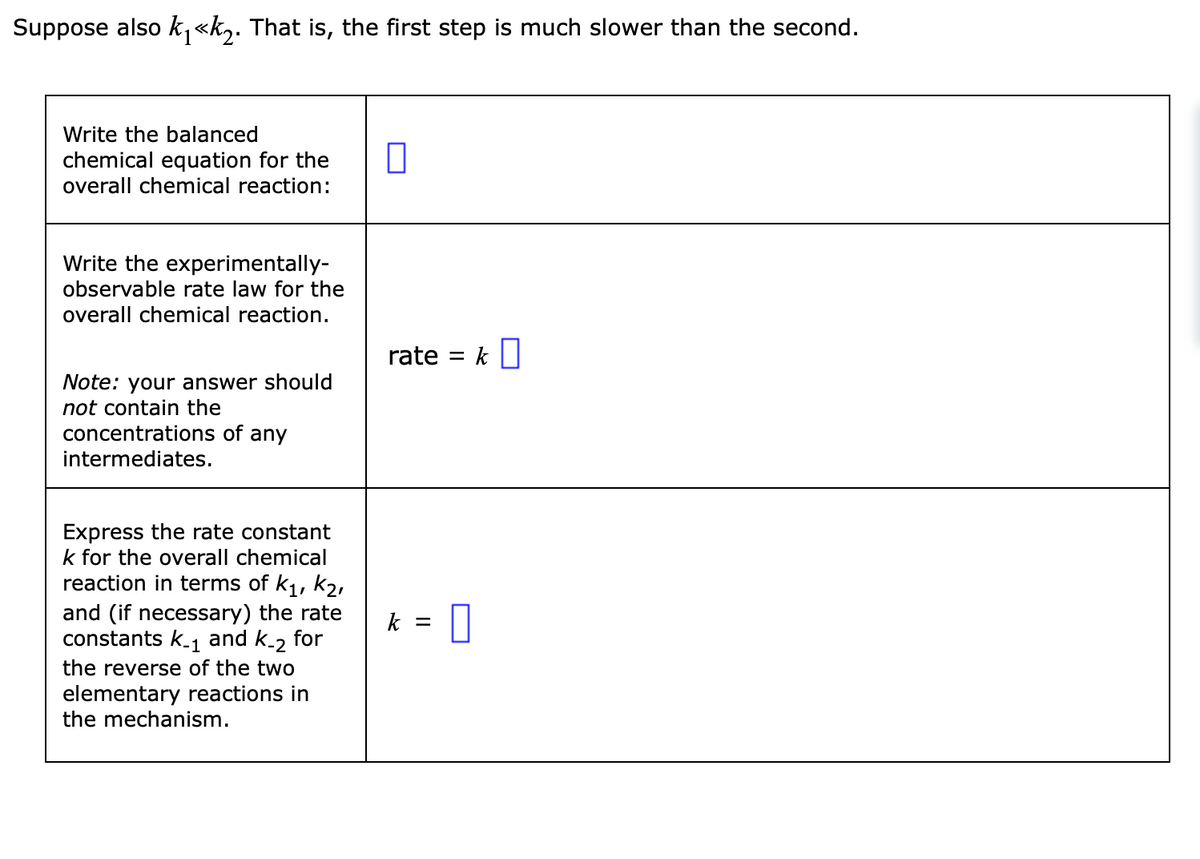
Transcribed Image Text:Suppose also k₁<<k₂. That is, the first step is much slower than the second.
Write the balanced
chemical equation for the
overall chemical reaction:
Write the experimentally-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of k₁, K₂,
and (if necessary) the rate
constants k_₁ and K-2 for
the reverse of the two
elementary reactions in
the mechanism.
1
0
rate = k
k = 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning