Suppose you build a galvanic cell using silver and chromium electrodes. a) Determine which electrode is the cathode and which is the anode under standard conditions (explain how you know). b) Write the half-reactions and the balanced complete reaction under standard conditions. c) Calculate the standard cell potential for your galvanic cell (walk through your work). d) Now suppose you have 0.100 M silver ions in the silver half-cell and 0.250 M chromium ions in the chromium half- cell. Calculate the cell potential under these conditions. Compare the answers for (c) and (d), and explain how the difference you see makes sense in terms of equilibrium.
Suppose you build a galvanic cell using silver and chromium electrodes. a) Determine which electrode is the cathode and which is the anode under standard conditions (explain how you know). b) Write the half-reactions and the balanced complete reaction under standard conditions. c) Calculate the standard cell potential for your galvanic cell (walk through your work). d) Now suppose you have 0.100 M silver ions in the silver half-cell and 0.250 M chromium ions in the chromium half- cell. Calculate the cell potential under these conditions. Compare the answers for (c) and (d), and explain how the difference you see makes sense in terms of equilibrium.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 60P
Related questions
Question
Part a,b,c,d properly solution

Transcribed Image Text:Suppose you build a galvanic cell using silver and chromium
electrodes.
a) Determine which electrode is the cathode and which is the
anode under standard conditions (explain how you know).
b) Write the half-reactions and the balanced complete
reaction under standard conditions.
c) Calculate the standard cell potential for your galvanic cell
(walk through your work).
d) Now suppose you have 0.100M silver ions in the silver
half-cell and 0.250 M chromium ions in the chromium half-
cell. Calculate the cell potential under these conditions.
Compare the answers for (c) and (d), and explain how the
difference you see makes sense in terms of equilibrium.
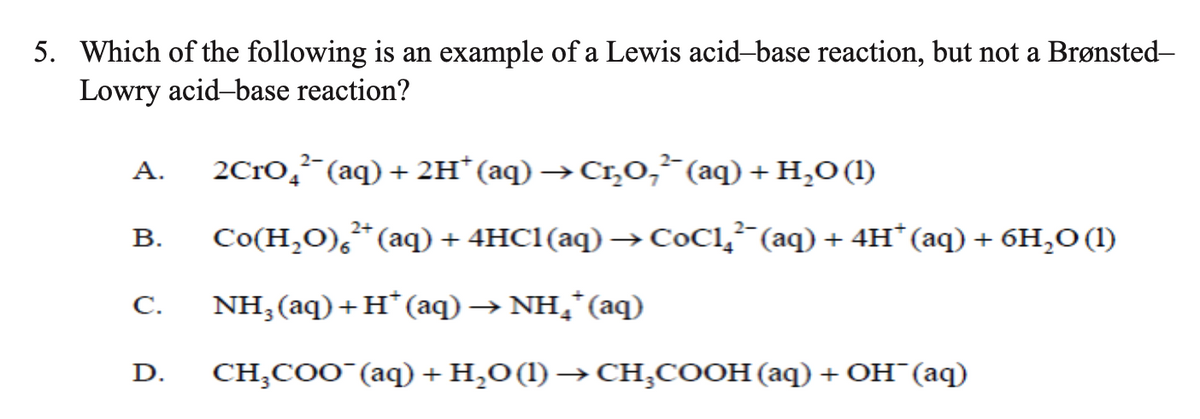
Transcribed Image Text:5. Which of the following is an example of a Lewis acid-base reaction, but not a Brønsted-
Lowry acid-base reaction?
А.
2C1O,²¯(aq) + 2H* (aq) →Cr,O, (aq) + H,O (1)
В.
Со(Н,0), * (аq) + 4HC1(aq) —> СОС1,;" (аq) + 4н" (аq) + бH,о()
C.
NH;(aq)+H*(aq) → NH,*(aq)
D.
CHCоо "(аq) + H,о(1) —СH,соон (аq) + Он (аq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
