Table 1: Estimation of Dissolved Oxygen: SI No Volume of water Initial sample (ml) burette Final burette Volume of NazS203 reading (ml) reading (ml) consumed (V2 ml) 100.0 0.0 7.4 7.4 1 100.0 7.4 14.8 7.4 2 100.0 14.8 22.1 7.3 3 Calculations: Normality of water sample: N¡V1(water sample) =N2V2(Na2S2O3) x74 N2 xV2 N1 = vi 40 1.85 x 10-3 100 N1 = 1.85 x 10-3 • Amount of Dissolved oxygen in water sample: Amount of Dissolved oxygen in water sample = N1 x equivalent weight of oxygen = 1.85 x 10-3 x 8 g/L 3D 1.85 х 10-3 х 8х 103 рpm = 14.8 PPM Amount of Dissolved oxygen in water sample = 14.8 PPM
Table 1: Estimation of Dissolved Oxygen: SI No Volume of water Initial sample (ml) burette Final burette Volume of NazS203 reading (ml) reading (ml) consumed (V2 ml) 100.0 0.0 7.4 7.4 1 100.0 7.4 14.8 7.4 2 100.0 14.8 22.1 7.3 3 Calculations: Normality of water sample: N¡V1(water sample) =N2V2(Na2S2O3) x74 N2 xV2 N1 = vi 40 1.85 x 10-3 100 N1 = 1.85 x 10-3 • Amount of Dissolved oxygen in water sample: Amount of Dissolved oxygen in water sample = N1 x equivalent weight of oxygen = 1.85 x 10-3 x 8 g/L 3D 1.85 х 10-3 х 8х 103 рpm = 14.8 PPM Amount of Dissolved oxygen in water sample = 14.8 PPM
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
EXPERIMENT :Estimation of Dissolved Oxygen
I want Result & Analysis for this experiment includes Precautions
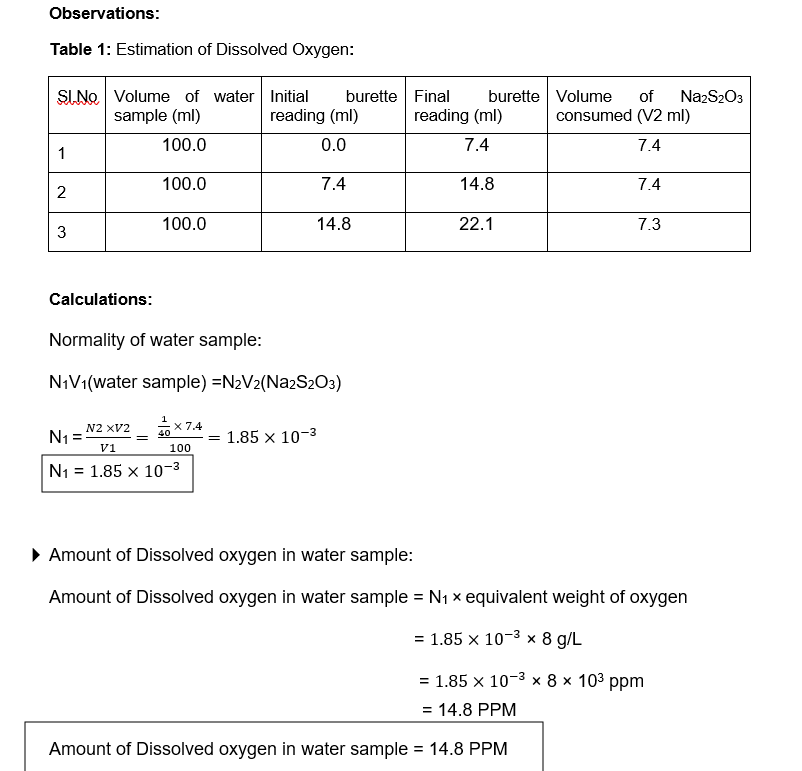
Transcribed Image Text:Observations:
Table 1: Estimation of Dissolved Oxygen:
SINO Volume of water Initial
sample (ml)
burette Final
burette Volume
of
NazS203
reading (ml)
reading (ml)
consumed (V2 ml)
100.0
0.0
7.4
7.4
1
100.0
7.4
14.8
7.4
2
100.0
14.8
22.1
7.3
3
Calculations:
Normality of water sample:
N¡Vi(water sample) =N2V2(Na2S203)
x 7.4
1.85 x 10-3
N2 XV2
N1 =
40
v1
100
N1 = 1.85 x 10-3
• Amount of Dissolved oxygen in water sample:
Amount of Dissolved oxygen in water sample = N1 x equivalent weight of oxygen
= 1.85 × 10-3 × 8 g/L
= 1.85 x 10-3 × 8 × 103 ppm
= 14.8 PPM
Amount of Dissolved oxygen in water sample = 14.8 PPM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning