Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.12: Dept 13c Nmr Spectroscopy
Problem 21P
Related questions
Question
I need help with analyzing this
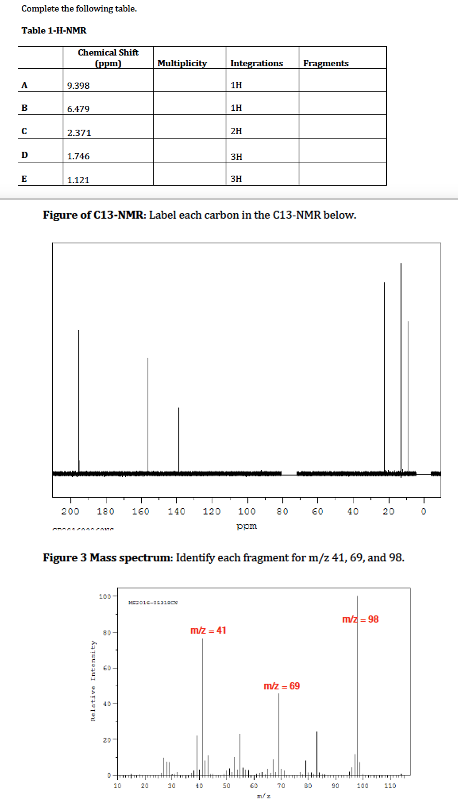
Transcribed Image Text:Complete the following table.
Table 1-H-NMR
Chemical Shift
(рpm)
Multiplicity
Integrations
Fragments
A
9.398
1H
в
6.479
1H
2.371
2H
D
1.746
3H
E
1.121
3H
Figure of C13-NMR: Label each carbon in the C13-NMR below.
200
180
160
140
120
100
80
60
40
20
ppm
Figure 3 Mass spectrum: Identify each fragment for m/z 41, 69, and 98.
MEe ame
m/z = 98
m/z = 41
mz = 69
40-
20
10
20
10
50
70
90
100
110
Relative Intensity
![Completely analyze the H-NMR, IR, C13 and mass spectrum to determine the
structure for the compound with chemical formula C6H100. Determine the 3
different fragments corresponding to the three different m/z shown below.
Calculate IHD. (Assign and label peaks to your proposed structure).
100
80
60
40
20
2000
Wavenumber [cm]
4000
3000
1500
1000
400
IR2016-90714TM
Wave number (cm') and Transmittance (T%)
2972 45 1689 5 1358 73 999 72
2937 59 1644 38 1306 75 867 81
2879 67 1461 68 1263 87 830 74
2818 73 1405 72 1221 56 774 88
2710 76 1380 81 1044 58 648 92
Clearly label and Draw structure here with labels for each peak in the H-NMR.
QE-300
200
100
c13 NMR
1H-NMR
A.
D](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F51435bf2-32bb-499c-aa57-a85dc35bd95d%2Fef01d953-e3e5-4824-9189-85dee3dabfa7%2Fwe7f9qn_processed.png&w=3840&q=75)
Transcribed Image Text:Completely analyze the H-NMR, IR, C13 and mass spectrum to determine the
structure for the compound with chemical formula C6H100. Determine the 3
different fragments corresponding to the three different m/z shown below.
Calculate IHD. (Assign and label peaks to your proposed structure).
100
80
60
40
20
2000
Wavenumber [cm]
4000
3000
1500
1000
400
IR2016-90714TM
Wave number (cm') and Transmittance (T%)
2972 45 1689 5 1358 73 999 72
2937 59 1644 38 1306 75 867 81
2879 67 1461 68 1263 87 830 74
2818 73 1405 72 1221 56 774 88
2710 76 1380 81 1044 58 648 92
Clearly label and Draw structure here with labels for each peak in the H-NMR.
QE-300
200
100
c13 NMR
1H-NMR
A.
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning