Table 2. Calculations ~60°C trial ~50°C trial ~40°C trial ~30°C trial ~20°C trial Vol. of HCI used (mL) 19.87 12.93 12.37 9.03 6.30 Moles of HCI 9.94 6.47 6.19 4.52 3.15 used (mol) Moles of borate present (mol) [Borate] (M) Ksp In(Ksp) 1/T(K-1)
Table 2. Calculations ~60°C trial ~50°C trial ~40°C trial ~30°C trial ~20°C trial Vol. of HCI used (mL) 19.87 12.93 12.37 9.03 6.30 Moles of HCI 9.94 6.47 6.19 4.52 3.15 used (mol) Moles of borate present (mol) [Borate] (M) Ksp In(Ksp) 1/T(K-1)
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 12P
Related questions
Question
Using the data collected in Table 1 fill in the blanks of Table 2
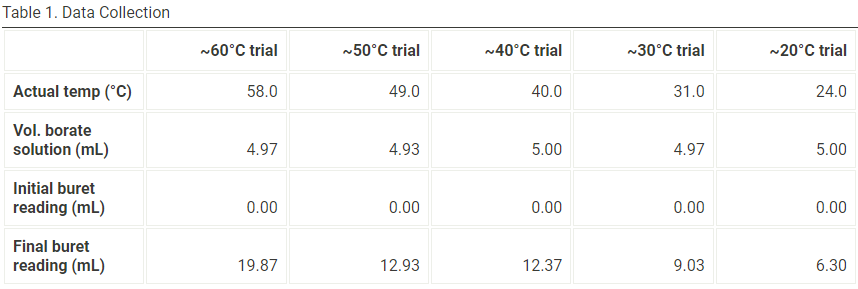
Transcribed Image Text:Table 1. Data Collection
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Actual temp (°C)
58.0
49.0
40.0
31.0
24.0
Vol. borate
solution (mL)
4.97
4.93
5.00
4.97
5.00
Initial buret
reading (mL)
0.00
0.00
0.00
0.00
0.00
Final buret
reading (mL)
19.87
12.93
12.37
9.03
6.30
![Table 2. Calculations
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Vol. of HCI used
(mL)
19.87
12.93
12.37
9.03
6.30
Moles of HCI
used (mol)
9.94
6.47
6.19
4.52
3.15
Moles of borate
present (mol)
[Borate] (M)
Ksp
In(Ksp)
1/T (K-1)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F108a0acc-c86a-4fb9-81b6-4d3403f04067%2Fbc3c55ae-ea6e-43a6-a27c-2467eb4ea72b%2F673fu5kjs_processed.png&w=3840&q=75)
Transcribed Image Text:Table 2. Calculations
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Vol. of HCI used
(mL)
19.87
12.93
12.37
9.03
6.30
Moles of HCI
used (mol)
9.94
6.47
6.19
4.52
3.15
Moles of borate
present (mol)
[Borate] (M)
Ksp
In(Ksp)
1/T (K-1)
Expert Solution
Step 1
In the given experiment the titration of borate with hydrochloric acid is being carried out at different temperatures. From the give data we are required to calculate the number of moles of borate titrated, concentration of borate at different temperature solubility product and the inverse of temperature.
Note: The number of moles of solution given are taken as millimoles instead of moles as it seems inappropriate to have 9.94 moles of in just of solution.
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



