Calculate the volume in milliliters of a 0.18M potassium dichromate solution that contains 25.0 g of potassium dichromate (K,Cr,o,). Be sure your answer has the correct number of significant digits. Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid relative strength Ka pK. П 2. x 10 (Choose one) 2.949 (Choose one) - 10 9.3 x 10 (Choose one)
Calculate the volume in milliliters of a 0.18M potassium dichromate solution that contains 25.0 g of potassium dichromate (K,Cr,o,). Be sure your answer has the correct number of significant digits. Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid relative strength Ka pK. П 2. x 10 (Choose one) 2.949 (Choose one) - 10 9.3 x 10 (Choose one)
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section14.2: Solution Concentration
Problem 13PP
Related questions
Question
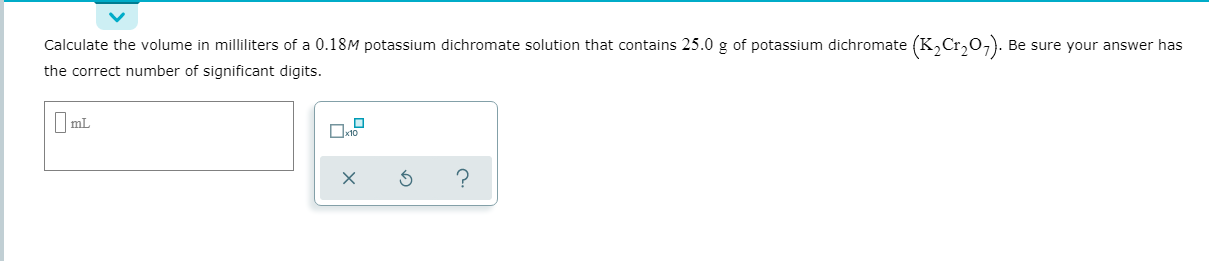
Transcribed Image Text:Calculate the volume in milliliters of a 0.18M potassium dichromate solution that contains 25.0 g of potassium dichromate (K,Cr,o,). Be sure your answer has
the correct number of significant digits.
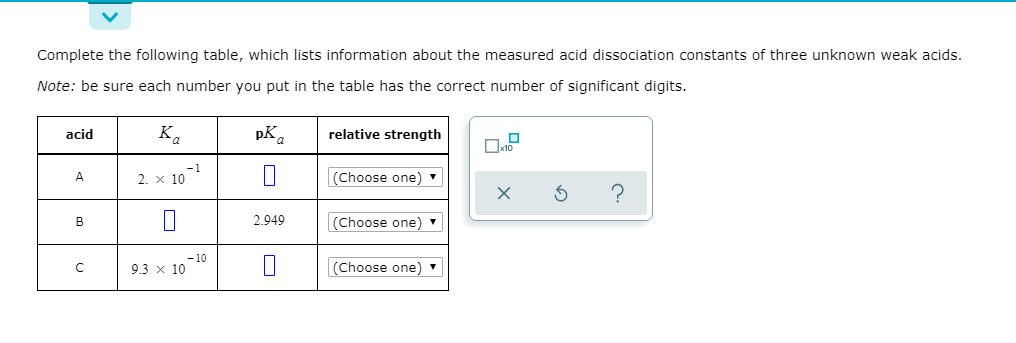
Transcribed Image Text:Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids.
Note: be sure each number you put in the table has the correct number of significant digits.
acid
relative strength
Ka
pK.
П
2. x 10
(Choose one)
2.949
(Choose one)
- 10
9.3 x 10
(Choose one)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning