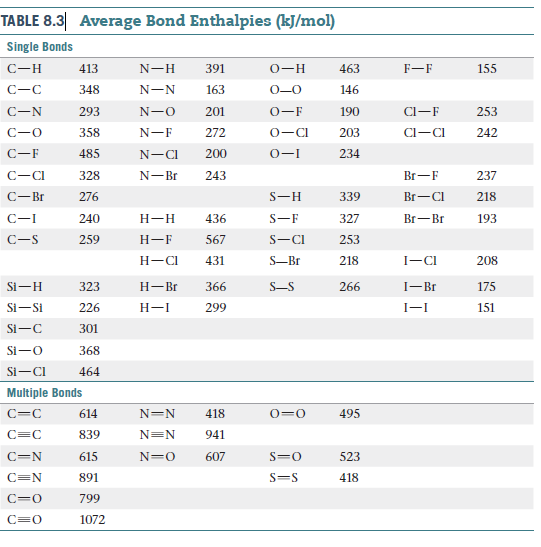
TABLE 8.3 Average Bond Enthalpies (kJ/mol) Single Bonds С—н 413 N-H 391 о—н 463 F-F 155 348 N-N 163 0-0 146 293 N-O 201 0-F 190 Cl-F 253 358 N-F 272 0-CI 203 Cl-CI 242 C-F 485 N-CI 200 0-I 234 C-CI 328 N-Br 243 Br-F 237 C-Br 276 339 Br-CI 218 C-I 240 Н-Н 436 327 Br-Br 193 C-S 259 Н—F 567 S-CI 253 Н- СI 431 S-Br 218 I-CI 208 Si-H 323 Н- Br 366 S-S 266 I-Br 175 Si-Si 226 Н-1 299 I-I 151 Si-C 301 Si-O 368 Si-CI 464 Multiple Bonds 614 N=N 418 0=0 495 839 N=N 941 615 607 523 C=N 891 418 799 1072
Atmospheric Pollution
In the atmosphere, the existence of large quantities of undesirable substances that could cause several health issues to living organisms and humans is atmospheric pollution. Air pollution is otherwise known to be atmospheric pollution. The presence of undesirable materials would also destruct the natural environment such as a change in climate, degradation of habitat, or depletion of ozone. Air pollution is generated by the natural processes and activities of humans.
Smokestack Scrubbers
Once we believe in the environment and remember the pollution-producing facets of it, we will consider the smoke-stacks scrubber to be a number of the worst offenders. Although this is often legally correct, smoke-stack scrubbers often have a big function in terms of keeping ground-level air pollutants- safe to breathe and assisting within the management of emissions.
The concentration of H2O in the stratosphere is about 5
ppm. It undergoes photodissociation according to:
H2O(g)----->H(g) + OH(g)
(a) Write out the Lewis-dot structures for both products and
reactant.
(b) Using Table , calculate the wavelength required to
cause this dissociation.
(c) The hydroxyl radical, OH, can react with ozone, giving
the following reactions:
OH (g) + O3(g)---->HO2(g) + O2(g)
HO2(g) + O(g)----->OH(g)+ O2(g)
What overall reaction results from these two elementary reactions?
What is the catalyst in the overall reaction? Explain.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 10 images









