Table of Vapor Pressures at Varying Temperatures Based on the Antoine Equation Pressure of Pressure of Pressure of 1-Butanol 1:1 (v/v) PROH/iPrQH PrQH/Butanol iPrQH/Butanol 1:1 (v/v) 1:1 (v/v) Temperature (C) 1-Propanol 2-Propanol (atm) (atm) (atm) 47 0.10215 0.19974 0.03816 50 0.11995 0.23293 0.04551 53 0.14035 0.27069 0.05406 56 0.16366 0.31351 0.06396 59 0.19022 0.36193 0.07538 62 0.22039 0.41652 0.08851 65 0.25455 0.47788 0.10355 68 0.29313 0.54666 0.12073 71 0.33658 0.62356 0.14029 74 0.38539 0.70931 0.16249 77 0.44008 0.80470 0.18762 80 0.50120 0.91055 0.21596 83 0.56934 1.02773 0.24786 86 0.64514 1.15717 0.28366 89 0.72927 1.29982 0.32373 92 0.82243 1.45671 0.36847 95 0.92538 1.62890 0.41831 98 1.03892 1.81751 0.47368 101 1.16387 2.02369 0.53507 104 1.30112 2.24866 0.60299 107 1.45159 2.49369 0.67795 110 1.61627 2.76009 0.76052 113 1.79615 3.04922 0.85130 116 1.99231 3.36250 0.95089 119 2.20585 3.70139 1.05995 122 2.43794 4.06740 1.17915 125 2.68978 4.46210 1.30921 Note: 1:1 (v/v) means one-to-one or equal parts of the components with respect to volumes; equal volumes of each component was used.
Table of Vapor Pressures at Varying Temperatures Based on the Antoine Equation Pressure of Pressure of Pressure of 1-Butanol 1:1 (v/v) PROH/iPrQH PrQH/Butanol iPrQH/Butanol 1:1 (v/v) 1:1 (v/v) Temperature (C) 1-Propanol 2-Propanol (atm) (atm) (atm) 47 0.10215 0.19974 0.03816 50 0.11995 0.23293 0.04551 53 0.14035 0.27069 0.05406 56 0.16366 0.31351 0.06396 59 0.19022 0.36193 0.07538 62 0.22039 0.41652 0.08851 65 0.25455 0.47788 0.10355 68 0.29313 0.54666 0.12073 71 0.33658 0.62356 0.14029 74 0.38539 0.70931 0.16249 77 0.44008 0.80470 0.18762 80 0.50120 0.91055 0.21596 83 0.56934 1.02773 0.24786 86 0.64514 1.15717 0.28366 89 0.72927 1.29982 0.32373 92 0.82243 1.45671 0.36847 95 0.92538 1.62890 0.41831 98 1.03892 1.81751 0.47368 101 1.16387 2.02369 0.53507 104 1.30112 2.24866 0.60299 107 1.45159 2.49369 0.67795 110 1.61627 2.76009 0.76052 113 1.79615 3.04922 0.85130 116 1.99231 3.36250 0.95089 119 2.20585 3.70139 1.05995 122 2.43794 4.06740 1.17915 125 2.68978 4.46210 1.30921 Note: 1:1 (v/v) means one-to-one or equal parts of the components with respect to volumes; equal volumes of each component was used.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 38GQ: The following data are the equilibrium vapor pressure of limonene, C10H16, at various temperatures....
Related questions
Question
How would I calculate the missing colums
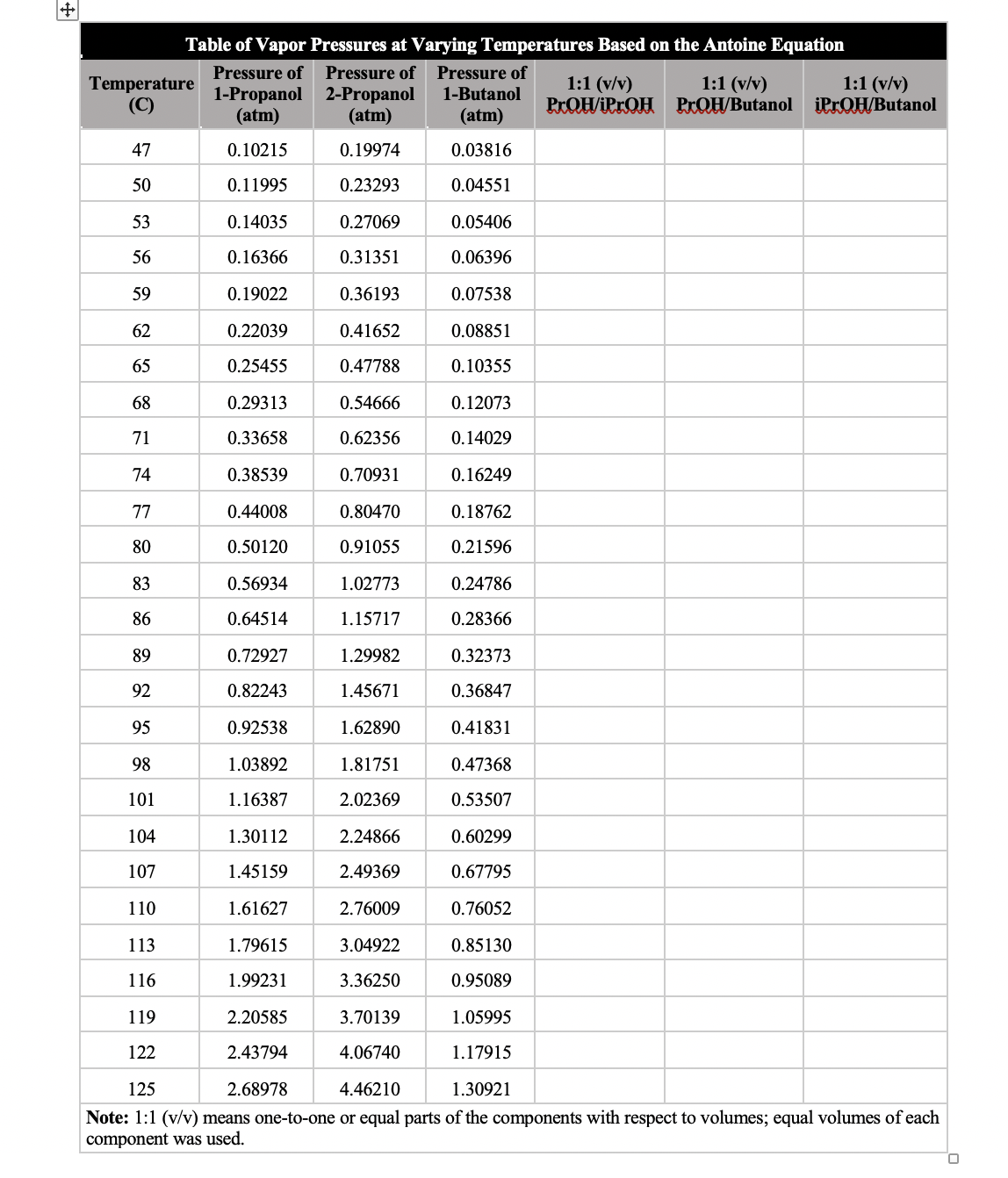
Transcribed Image Text:Table of Vapor Pressures at Varying Temperatures Based on the Antoine Equation
Pressure of
Pressure of
Pressure of
1:1 (v/v)
Temperature
(C)
1-Propanol 2-Propanol
(atm)
1:1 (v/v)
PROH/iPrQH PrQH/Butanol
1:1 (v/v)
iPrOH/Butanol
1-Butanol
(atm)
(atm)
47
0.10215
0.19974
0.03816
50
0.11995
0.23293
0.04551
53
0.14035
0.27069
0.05406
56
0.16366
0.31351
0.06396
59
0.19022
0.36193
0.07538
62
0.22039
0.41652
0.08851
65
0.25455
0.47788
0.10355
68
0.29313
0.54666
0.12073
71
0.33658
0.62356
0.14029
74
0.38539
0.70931
0.16249
77
0.44008
0.80470
0.18762
80
0.50120
0.91055
0.21596
83
0.56934
1.02773
0.24786
86
0.64514
1.15717
0.28366
89
0.72927
1.29982
0.32373
92
0.82243
1.45671
0.36847
95
0.92538
1.62890
0.41831
98
1.03892
1.81751
0.47368
101
1.16387
2.02369
0.53507
104
1.30112
2.24866
0.60299
107
1.45159
2.49369
0.67795
110
1.61627
2.76009
0.76052
113
1.79615
3.04922
0.85130
116
1.99231
3.36250
0.95089
119
2.20585
3.70139
1.05995
122
2.43794
4.06740
1.17915
125
2.68978
4.46210
1.30921
Note: 1:1 (v/v) means one-to-one or equal parts of the components with respect to volumes; equal volumes of each
component was used.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole