The graph of vapor pressure vs. temperature for three liquids, trichloromethane, tetrachloromethane and water, are shown below. CHCI, cC4 H20 1000 800- 60아 400 200 O 20 40 60 80 Temperature (°C) Estimate the temperatures at which the vapor pressure of CCl is 400. torr and 700. torr. (The dash lines are for your reference.) Use these values to calculat the enthalpy of vaporization, AHvap, of CCl. Using information provided in the graph, rank the overall strength of intermolecular forces in the three liquids, from the weakest to the strongest. Describe how you know. What intermolecular forces are present in liquid CHCI3 and in liquid CCl4? Describe how you know from the molecular structural level. Vapor Pressure (tom)
The graph of vapor pressure vs. temperature for three liquids, trichloromethane, tetrachloromethane and water, are shown below. CHCI, cC4 H20 1000 800- 60아 400 200 O 20 40 60 80 Temperature (°C) Estimate the temperatures at which the vapor pressure of CCl is 400. torr and 700. torr. (The dash lines are for your reference.) Use these values to calculat the enthalpy of vaporization, AHvap, of CCl. Using information provided in the graph, rank the overall strength of intermolecular forces in the three liquids, from the weakest to the strongest. Describe how you know. What intermolecular forces are present in liquid CHCI3 and in liquid CCl4? Describe how you know from the molecular structural level. Vapor Pressure (tom)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 17E
Related questions
Question
Need help with a Chemistry Problem! Unsure how to complete this practice Problem
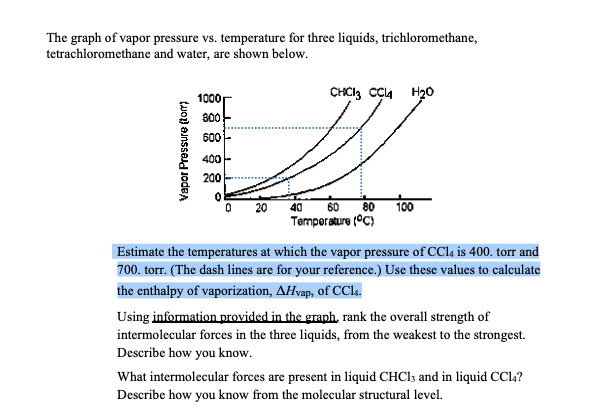
Transcribed Image Text:The graph of vapor pressure vs. temperature for three liquids, trichloromethane,
tetrachloromethane and water, are shown below.
1000
CHCI, C4 H20
800
600
400 -
200
60 80 100
Temporature (°C)
20
40
Estimate the temperatures at which the vapor pressure of CC14 is 400. torr and
700. torr. (The dash lines are for your reference.) Use these values to calculate
the enthalpy of vaporization, AHvap, of CCla.
Using information provided in the graph, rank the overall strength of
intermolecular forces in the three liquids, from the weakest to the strongest.
Describe how you know.
What intermolecular forces are present in liquid CHCI; and in liquid CCl,?
Describe how you know from the molecular structural level.
Vapor Pressure (tom)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning